scale and corrosion inhibitor for cooling tower
Scale and Corrosion Inhibitors for Cooling Towers
Cooling towers play a crucial role in various industrial processes, providing an essential mechanism for heat dissipation. However, the operation of these systems can be adversely affected by two common issues scale formation and corrosion. Both phenomena can significantly reduce the efficiency and lifespan of cooling towers, leading to costly downtime and repairs. Consequently, the implementation of scale and corrosion inhibitors is critical for maintaining optimal performance and extending the service life of these systems.
Understanding Scale Formation
Scale is primarily composed of mineral deposits, such as calcium carbonate, calcium sulfate, and magnesium silicate. These deposits occur when water evaporates in the cooling tower, leading to a concentrated solution of minerals that precipitate out of the water due to oversaturation. As these deposits accumulate on heat exchange surfaces, they reduce heat transfer efficiency, resulting in increased energy costs and potential overheating of equipment.
Scale formation is influenced by several factors, including water chemistry, temperature, and pH levels. Hard water, characterized by high concentrations of dissolved minerals, is particularly prone to scaling. Furthermore, operational conditions such as high evaporation rates and temperature fluctuations can exacerbate scale development, making it imperative to monitor and control these factors actively.
Corrosion Concerns
Corrosion is the gradual degradation of materials, often metal, due to chemical reactions with their environment. In cooling towers, corrosion can occur when water interacts with metallic components, leading to the formation of rust or other corrosion products. Factors contributing to corrosion include the presence of oxygen, low pH conditions, and the presence of aggressive ions such as chlorides.
Corrosion not only weakens structural integrity but also leads to leaks and further operational inefficiencies. The economic implications are significant maintenance costs can escalate quickly if corrosion is not effectively managed. Moreover, corrosion can lead to system failures, resulting in unscheduled downtime that disrupts operations and increases costs.
The Role of Inhibitors
To mitigate the adverse effects of scale and corrosion, operators often rely on chemical inhibitors. These substances are added to the cooling water to prevent or reduce the formation of scale and the occurrence of corrosion. The choice of inhibitor depends on specific water chemistry, operational conditions, and the types of materials used in the cooling tower.
Scale Inhibitors These agents function by interfering with the crystallization process of scale-forming minerals. Common scale inhibitors include phosphonates, polyacrylic acids, and organic derivatives. By modifying the surface properties of scaling minerals, these inhibitors help to keep them suspended in the water rather than allowing them to settle on surfaces. This ultimately leads to fewer maintenance issues and improved heat transfer efficiency.
scale and corrosion inhibitor for cooling tower
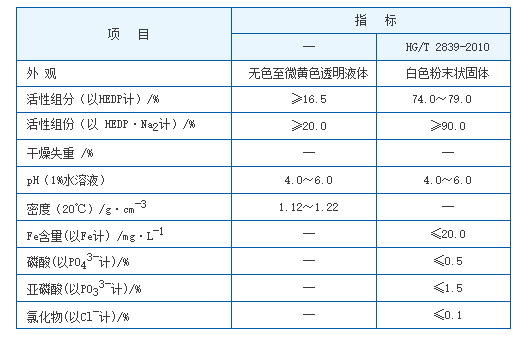
Corrosion Inhibitors The function of corrosion inhibitors is to create a protective layer on metal surfaces, reducing their reactivity with water and other corrosive agents. Common types of corrosion inhibitors include film-forming compounds, which deposit a protective coating on surfaces. Additionally, anodic inhibitors can help to passivate metal surfaces, decreasing corrosion rates significantly. The selection of a corrosion inhibitor usually depends on factors such as the metal substrate, the environment, and the presence of specific contaminants.
Best Practices for Inhibitor Application
To ensure that scale and corrosion inhibitors are effective, it's essential to implement best practices in their application
1. Regular Monitoring Continuous monitoring of water chemistry, including hardness, alkalinity, pH, and conductivity, is vital for identifying potential scaling and corrosion risks early on.
2. Tailored Inhibitor Selection Different systems and water chemistries may require specific types of inhibitors. Conducting a comprehensive analysis can help in selecting the most suitable products.
3. Proper Dosing The effectiveness of inhibitors largely depends on correct dosing. Operators should adhere to manufacturer guidelines and adjust concentrations based on system conditions.
4. System Maintenance Regular inspection and maintenance of the cooling tower system are critical. This includes cleaning and descaling as needed, as well as checking for any signs of corrosion.
5. Training and Education Ensuring that staff responsible for water treatment are well-trained can significantly enhance the effectiveness of inhibitor programs.
Conclusion
In conclusion, the application of scale and corrosion inhibitors is vital for the efficient and prolonged operation of cooling towers. By addressing these common issues, industries can decrease maintenance costs, enhance operational efficiency, and prolong the lifespan of cooling systems. Optimal water management strategies, combined with the right chemical treatments, form the backbone of successful cooling tower operations in today’s industrial landscape.
-
Understanding Polycarboxylic Acids: Properties, Applications, and Future PotentialNewsJul.28,2025
-
Scale Inhibitor Explained: How to Protect Your System from Limescale and Hard Water DamageNewsJul.28,2025
-
Scale and Corrosion Inhibitors: Essential Chemicals for Industrial Water System ProtectionNewsJul.28,2025
-
Polyaspartic Acid: A Biodegradable Polymer for Sustainable ChemistryNewsJul.28,2025
-
Isothiazolinones: A Versatile Antimicrobial Class with Industrial Power and Regulatory ChallengesNewsJul.28,2025
-
A Deep Dive into 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTC)NewsJul.28,2025





