pbtc tricarboxylic acid
Understanding the Role of Pyruvate in the Tricarboxylic Acid Cycle
The tricarboxylic acid cycle (TCA cycle), also known as the Krebs cycle or citric acid cycle, is a vital metabolic pathway that occurs within the mitochondria of eukaryotic cells. This complex series of biochemical reactions plays a crucial role in energy production, cellular respiration, and the metabolism of various biomolecules. At the heart of this intricate process lies pyruvate, a key intermediary that bridges glycolysis and the TCA cycle.
The Formation of Pyruvate
Pyruvate is generated during glycolysis, the first stage of cellular respiration, where glucose is broken down into two molecules of pyruvate. This process, which occurs in the cytoplasm, is anaerobic, meaning it does not require oxygen. Once formed, pyruvate can take several pathways depending on the availability of oxygen and the cellular conditions. Under aerobic conditions, pyruvate is transported into the mitochondria, where it undergoes decarboxylation, catalyzed by the pyruvate dehydrogenase complex. This reaction converts pyruvate into acetyl-CoA, releasing carbon dioxide and producing NADH, an important electron carrier.
Pyruvate’s Entry into the TCA Cycle
Understanding the Role of Pyruvate in the Tricarboxylic Acid Cycle
As acetyl-CoA enters the TCA cycle, it undergoes a series of transformations. It is first converted to citrate and then isomerized to isocitrate. Isocitrate is subsequently oxidized and decarboxylated to alpha-ketoglutarate, which is another key junction in cellular metabolism. This step is critical because it produces another molecule of NADH and releases carbon dioxide, a waste product that needs to be eliminated from the organism.
pbtc tricarboxylic acid
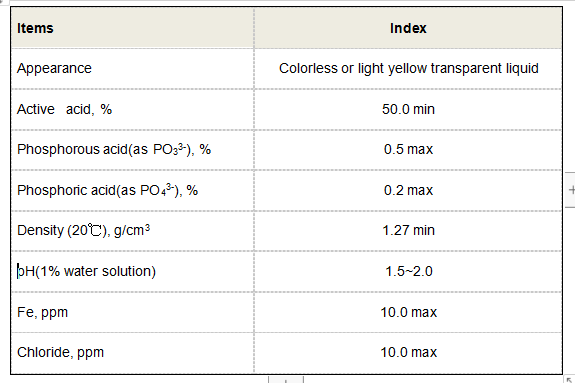
The Importance of Pyruvate in Cellular Metabolism
Pyruvate's significance extends beyond its conversion into acetyl-CoA. It serves as a versatile metabolic hub that can lead to various biochemical pathways. In low-oxygen conditions, pyruvate can be converted into lactate through anaerobic fermentation, enabling the recycling of NAD+ necessary for glycolysis to continue. This adaptability allows cells to vary their metabolic processes based on the availability of oxygen and energy demands.
Moreover, pyruvate can also be utilized in gluconeogenesis, the synthesis of glucose from non-carbohydrate precursors. This pathway is especially important in maintaining blood sugar levels during prolonged fasting or intense exercise. Additionally, pyruvate plays a role in the synthesis of fatty acids and amino acids, showcasing its versatility in different metabolic contexts.
Conclusion
In summary, pyruvate is a pivotal compound in cellular metabolism, linking the pathways of glycolysis and the TCA cycle. Its conversion to acetyl-CoA marks a significant transition where energy production shifts from anaerobic to aerobic respiration. The TCA cycle, driven by the presence of acetyl-CoA, continuously oxidizes substrates to generate energy-rich molecules essential for cellular functions.
Understanding the role of pyruvate not only highlights the intricate workings of metabolic pathways but also emphasizes the adaptability of cellular metabolism in response to different energy demands. In a broader context, this understanding can provide insights into metabolic disorders, energy imbalances, and potential therapeutic strategies aimed at regulating these fundamental biochemical processes. The study of pyruvate and its implications in the TCA cycle continues to be an important area in both basic and applied biological research, underscoring the complexity and efficiency of metabolic regulation in living organisms.
-
2 Phosphonobutane 1,2,4 Tricarboxylic Acid (PBTCA): Superior Scale & Corrosion InhibitorNewsAug.31,2025
-
Dodecyldimethylbenzylammonium Chloride: High-Purity DisinfectantNewsAug.30,2025
-
2-Phosphonobutane-1,2,4-Tricarboxylic Acid: Scale & CorrosionNewsAug.29,2025
-
Premium Isothiazolinones | Broad-Spectrum Biocidal SolutionsNewsAug.28,2025
-
LK-319 Special Scale And Corrosion Inhibitor For Steel Plants: Advanced Solutions for Industrial Water SystemsNewsAug.22,2025
-
Flocculant Water Treatment: Essential Chemical Solutions for Purification ProcessesNewsAug.22,2025





