Exploring the Relationship Between Scale Formation and Corrosion Inhibitor Chemical Effectiveness in Industrial Processes
Scale and Corrosion Inhibitor Chemicals An Overview
In various industrial applications, particularly those involving heat exchangers, boilers, and cooling towers, scale and corrosion are two of the most significant challenges faced by engineers and facility managers. Scale is primarily the result of mineral deposits from water, while corrosion arises from chemical reactions between metal surfaces and their environment. Both phenomena can lead to substantial operational inefficiencies, increased maintenance costs, and potentially catastrophic failures if not addressed. To combat these issues, scale and corrosion inhibitor chemicals are widely used across different sectors.
Understanding Scale Formation
Scale is formed when dissolved minerals, such as calcium and magnesium, exceed their solubility limits and precipitate out of solution, often due to temperature changes or evaporation. This deposit tends to accumulate over time on surfaces, leading to reduced heat transfer efficiency and increased energy consumption. For instance, in a boiler, scale can act as an insulating layer, which forces the system to work harder to achieve the desired temperature, resulting in higher fuel costs and the potential for overheating.
To mitigate scale formation, various chemicals are employed. Phosphonates, polyacrylates, and organic acids are among the most commonly used scale inhibitors. They function by interfering with the crystal growth of mineral deposits, effectively preventing them from adhering to surfaces. Additionally, similar agents can change the morphology of scale crystals, making them less likely to stick or facilitating their removal through regular water flow.
Corrosion Causes and Impacts
Corrosion, on the other hand, is an electrochemical process that deteriorates metal surfaces, leading to pitting, rusting, and ultimately, structural failure. The presence of oxygen, moisture, and aggressive ions such as chlorides in the environment significantly accelerates this process. In industrial settings, corrosion can lead to leaks, failures, and unsafe operating conditions, emphasizing the need for effective corrosion control measures.
scale and corrosion inhibitor chemicals
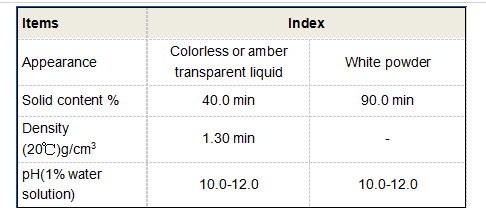
Corrosion inhibitors are chemicals that reduce the rate of corrosion by forming a protective film over metal surfaces or by reacting with corrosive elements to neutralize them. Common types of corrosion inhibitors include anodic inhibitors, cathodic inhibitors, and volatile corrosion inhibitors (VCIs). Each type works through different mechanisms anodic inhibitors tend to reduce the oxidation process at the anode, while cathodic inhibitors reduce the reduction reaction at the cathode. VCIs, often used in packaging, release vapor that condenses onto metal surfaces, providing a protective barrier.
Applications and Best Practices
The selection and application of scale and corrosion inhibitors depend on several factors, including the specific system involved, the type of water used, and the operating conditions. In cooling systems, for instance, a comprehensive treatment program typically includes both scale and corrosion inhibitors. This integrated approach minimizes the adverse effects of both scale and corrosion simultaneously, enhancing system efficiency and longevity.
Moreover, monitoring and maintaining appropriate chemical concentrations are crucial for the effectiveness of these inhibitors. Regular water analyses help ensure that the chemicals remain within optimal ranges, preventing the resurgence of scale and corrosion issues.
Conclusion
In summary, scale and corrosion inhibitors play a pivotal role in maintaining the efficiency and safety of industrial systems. Understanding the nature of scale and corrosion and employing the right chemicals can lead to significant improvements in operational performance, reduced downtime, and long-term cost savings. As industries continue to innovate and evolve, the development of more effective and environmentally friendly inhibitors will be vital in addressing these persistent challenges.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





