Investigation on the Formation Mechanism of Polyacrylamide and Its Applications
Understanding Polyacrylamide Formation A Comprehensive Overview
Polyacrylamide (PAM) is a synthetic polymer widely used for various applications due to its unique properties, such as high water solubility, gel formation, and viscoelasticity. Its formation primarily involves the polymerization of acrylamide monomers, leading to a versatile compound that plays an integral role in fields ranging from water treatment to agriculture.
The Formation Process
The formation of polyacrylamide occurs through a chemical reaction called radical polymerization. This process begins with the initiation of acrylamide, typically in an aqueous solution, where various initiators such as potassium persulfate or ammonium persulfate are used. These initiators decompose thermally to produce free radicals, creating a reactive environment that promotes the linking of acrylamide monomers.
The reaction proceeds through several steps
1. Initiation A free radical is generated by the thermal decomposition of the initiator, which reacts with the acrylamide monomer to form an active site.
2. Propagation The active site generated from the first step reacts with another acrylamide molecule, creating a dimer. This step continues, leading to the formation of longer chains as more acrylamide monomers are added.
3. Termination Eventually, the growth of the polymer chains reaches a point of termination. This can occur through various mechanisms, such as chain transfer or coupling reactions, leading to the formation of polyacrylamide in its final form.
Factors Influencing Polyacrylamide Formation
Several factors influence the properties and structure of the resulting polyacrylamide
1. Concentration of Acrylamide The ratio of acrylamide monomers to water and other reactants affects the viscosity and density of the final product. Higher concentrations can lead to more entangled networks, resulting in gels with varying strengths.
polyacrylamide formation
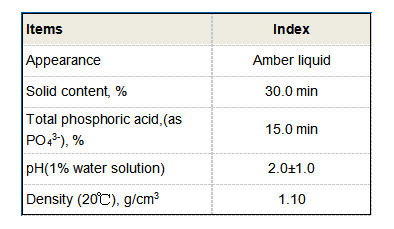
2. Temperature The polymerization process is temperature-dependent. Higher temperatures may increase the rate of reaction, but they can also affect the stability of the polymer chains formed, potentially leading to degradation.
3. pH Levels The pH of the reaction medium can influence the rate of polymerization and the characteristics of the final product. Basic conditions often facilitate a faster reaction rate, while acidic conditions could result in slower formation and modifications in the polymer's properties.
4. Presence of Crosslinkers Introducing crosslinking agents can transform polyacrylamide into a gel-like structure. Crosslinking enhances the stability and enhances the application potential of PAM in various fields, particularly in the creation of hydrogels used for drug delivery and tissue engineering.
Applications of Polyacrylamide
Polyacrylamide has found extensive application across numerous fields due to its favorable properties
1. Water Treatment PAM is heavily utilized in water treatment plants to enhance the settling of solids and improve water clarity. It works by flocculating suspended particles, making them easier to remove.
2. Agriculture In soil management, polyacrylamide is used to reduce erosion and improve water retention in arable land. It helps maintain soil moisture, enhancing crop yield while minimizing water usage.
3. Oil Recovery The oil industry employs polyacrylamide in enhanced oil recovery operations. The polymer helps to increase the viscosity of water injected into oil reservoirs, improving the displacement of oil trapped in porous media.
4. Biomedical Applications Polyacrylamide gels are commonly used in laboratories for electrophoresis, particularly in DNA separation and analysis, due to their ability to create uniform pore sizes.
Conclusion
The formation of polyacrylamide is a significant chemical process with far-reaching implications across multiple sectors. By understanding the variables that affect its synthesis and structure, researchers and industries can better tailor its properties for specific applications. From environmental management to cutting-edge biomedical uses, polyacrylamide remains a critical compound whose versatility continues to inspire innovation in science and technology. Understanding its formation is vital for leveraging its full potential in addressing global challenges.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





