scale and corrosion inhibitor
Understanding Scale Formation and the Role of Corrosion Inhibitors in Industrial Applications
In various industrial processes, particularly in water systems, the presence of scale can pose significant challenges. Scale formation, primarily composed of mineral deposits like calcium carbonate and magnesium sulfate, occurs when water is heated or evaporated, leading to crystallization. This phenomenon not only impedes the efficiency of heat exchangers and cooling systems but also increases maintenance costs and energy consumption. To mitigate these issues, corrosion inhibitors play a crucial role in controlling scale formation while preserving the integrity of the equipment.
Scale formation arises from the precipitation of dissolved minerals as water temperature rises or as pressure changes occur in pipelines and vessels. In industries such as oil and gas, power generation, and water treatment, the resulting scale can clog pipes, degrade heat transfer efficiency, and ultimately lead to equipment failure. This not only disrupts operations but also poses significant safety risks.
Understanding Scale Formation and the Role of Corrosion Inhibitors in Industrial Applications
Corrosion inhibitors are chemical substances that, when added to a system, reduce the rate of corrosion of metals. They work by forming a protective film on metal surfaces, inhibiting the electrochemical reactions that lead to corrosion. Common types of corrosion inhibitors include anodic inhibitors, cathodic inhibitors, and passivators. The choice of inhibitor depends on the specific water chemistry, temperature, and flow conditions of the system.
scale and corrosion inhibitor
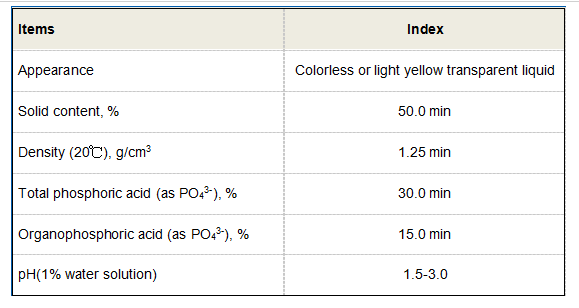
One effective strategy for controlling both scale and corrosion simultaneously is the use of combined treatments. For instance, certain organic phosphonates can act as both scale inhibitors and corrosion inhibitors, providing a dual action that enhances overall system protection. These compounds work by complexing with mineral ions in the water, reducing their ability to precipitate while also forming a protective layer on metal surfaces.
In addition to chemical treatments, operational changes can also contribute to scale and corrosion management. For example, controlling the temperature and pressure of a system can help minimize scale deposition. Implementing regular monitoring of water chemistry allows operators to adjust treatment levels proactively, ensuring optimal performance of corrosion inhibitors and scale control agents.
While the focus on scale and corrosion may highlight the challenges faced by industrial operators, it also underscores the importance of continued research and innovation in treatment solutions. Advancements in nanotechnology, for instance, offer promising avenues for creating more effective inhibitors with fewer environmental impacts. By developing multifunctional materials that can address both issues, researchers are paving the way for more sustainable industrial practices.
In conclusion, scale formation and corrosion are intertwined challenges that demand a comprehensive approach in industrial applications. The role of corrosion inhibitors is vital in mitigating the adverse effects of scale while protecting equipment. By utilizing combined treatments and employing effective operational strategies, industries can enhance the longevity and efficiency of their systems. As technology continues to evolve, the pursuit of improved scale and corrosion management will play a crucial role in ensuring sustainable industrial growth and operational safety. The commitment to innovation in this field not only benefits individual enterprises but also contributes to broader environmental stewardship and resource conservation.
-
Dodecyldimethylbenzylammonium Chloride: High-Purity DisinfectantNewsAug.30,2025
-
2-Phosphonobutane-1,2,4-Tricarboxylic Acid: Scale & CorrosionNewsAug.29,2025
-
Premium Isothiazolinones | Broad-Spectrum Biocidal SolutionsNewsAug.28,2025
-
LK-319 Special Scale And Corrosion Inhibitor For Steel Plants: Advanced Solutions for Industrial Water SystemsNewsAug.22,2025
-
Flocculant Water Treatment: Essential Chemical Solutions for Purification ProcessesNewsAug.22,2025
-
Isothiazolinones: Versatile Microbial Control Agents for Industrial and Consumer ApplicationsNewsAug.22,2025





