6月 . 28, 2024 12:30
Back to list
A novel approach to the synthesis of polyaspartic acid sodium salt
Sodium Polyaspartate A Versatile and Effective Water Treatment Agent
Sodium polyaspartate, a versatile polymer derived from aspartic acid, has emerged as a highly effective water treatment agent. This compound is known for its ability to chelate various metal ions, making it particularly useful in the removal of heavy metals from water sources.
Chelation is a process in which a molecule or ion forms a stable complex with another molecule or ion, often involving the donation of electrons. In the case of sodium polyaspartate, its ability to chelate metal ions is due to the presence of carboxyl groups (-COOH) in its molecular structure. These carboxyl groups have a high affinity for metal ions, allowing them to form strong bonds and effectively remove these contaminants from water.
One of the key advantages of using sodium polyaspartate for water treatment is its environmental friendliness. Unlike other chelating agents that may contain toxic substances, sodium polyaspartate is biodegradable and non-toxic, making it safe for both the environment and human health. This makes it an attractive alternative for treating drinking water and industrial wastewater.
Another important aspect of sodium polyaspartate's effectiveness as a water treatment agent is its ability to remove a wide range of metal ions. This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel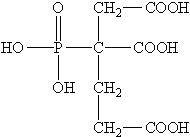 This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel
This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel sodium of polyaspartic acid. By forming stable complexes with these ions, sodium polyaspartate can effectively reduce their concentrations in water to levels that meet regulatory standards.
Furthermore, sodium polyaspartate exhibits excellent performance in terms of its ability to prevent scale formation. Scale is a common problem in water systems, especially in boilers and cooling towers, where it can cause significant damage and reduce efficiency. Sodium polyaspartate works by interfering with the nucleation and growth of scale-forming minerals, such as calcium carbonate and magnesium hydroxide. By doing so, it helps to maintain the cleanliness and efficiency of water systems over time.
In conclusion, sodium polyaspartate is a powerful and versatile water treatment agent that offers numerous benefits for both environmental protection and industrial applications. Its ability to chelate metal ions, coupled with its biodegradability and non-toxicity, make it an attractive alternative to traditional water treatment methods. As research continues to explore its potential uses and applications, it is likely that we will see even more widespread adoption of this remarkable compound in the future.
sodium of polyaspartic acid. By forming stable complexes with these ions, sodium polyaspartate can effectively reduce their concentrations in water to levels that meet regulatory standards.
Furthermore, sodium polyaspartate exhibits excellent performance in terms of its ability to prevent scale formation. Scale is a common problem in water systems, especially in boilers and cooling towers, where it can cause significant damage and reduce efficiency. Sodium polyaspartate works by interfering with the nucleation and growth of scale-forming minerals, such as calcium carbonate and magnesium hydroxide. By doing so, it helps to maintain the cleanliness and efficiency of water systems over time.
In conclusion, sodium polyaspartate is a powerful and versatile water treatment agent that offers numerous benefits for both environmental protection and industrial applications. Its ability to chelate metal ions, coupled with its biodegradability and non-toxicity, make it an attractive alternative to traditional water treatment methods. As research continues to explore its potential uses and applications, it is likely that we will see even more widespread adoption of this remarkable compound in the future.
 This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel
This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel This includes not only heavy metals such as lead, mercury, and cadmium but also transition metals like copper, zinc, and nickel sodium of polyaspartic acid. By forming stable complexes with these ions, sodium polyaspartate can effectively reduce their concentrations in water to levels that meet regulatory standards.
Furthermore, sodium polyaspartate exhibits excellent performance in terms of its ability to prevent scale formation. Scale is a common problem in water systems, especially in boilers and cooling towers, where it can cause significant damage and reduce efficiency. Sodium polyaspartate works by interfering with the nucleation and growth of scale-forming minerals, such as calcium carbonate and magnesium hydroxide. By doing so, it helps to maintain the cleanliness and efficiency of water systems over time.
In conclusion, sodium polyaspartate is a powerful and versatile water treatment agent that offers numerous benefits for both environmental protection and industrial applications. Its ability to chelate metal ions, coupled with its biodegradability and non-toxicity, make it an attractive alternative to traditional water treatment methods. As research continues to explore its potential uses and applications, it is likely that we will see even more widespread adoption of this remarkable compound in the future.
sodium of polyaspartic acid. By forming stable complexes with these ions, sodium polyaspartate can effectively reduce their concentrations in water to levels that meet regulatory standards.
Furthermore, sodium polyaspartate exhibits excellent performance in terms of its ability to prevent scale formation. Scale is a common problem in water systems, especially in boilers and cooling towers, where it can cause significant damage and reduce efficiency. Sodium polyaspartate works by interfering with the nucleation and growth of scale-forming minerals, such as calcium carbonate and magnesium hydroxide. By doing so, it helps to maintain the cleanliness and efficiency of water systems over time.
In conclusion, sodium polyaspartate is a powerful and versatile water treatment agent that offers numerous benefits for both environmental protection and industrial applications. Its ability to chelate metal ions, coupled with its biodegradability and non-toxicity, make it an attractive alternative to traditional water treatment methods. As research continues to explore its potential uses and applications, it is likely that we will see even more widespread adoption of this remarkable compound in the future. Share
Next:
Latest news
-
Understanding Polycarboxylic Acids: Properties, Applications, and Future PotentialNewsJul.28,2025
-
Scale Inhibitor Explained: How to Protect Your System from Limescale and Hard Water DamageNewsJul.28,2025
-
Scale and Corrosion Inhibitors: Essential Chemicals for Industrial Water System ProtectionNewsJul.28,2025
-
Polyaspartic Acid: A Biodegradable Polymer for Sustainable ChemistryNewsJul.28,2025
-
Isothiazolinones: A Versatile Antimicrobial Class with Industrial Power and Regulatory ChallengesNewsJul.28,2025
-
A Deep Dive into 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTC)NewsJul.28,2025





