flocculation chemicals
Flocculation Chemistry Understanding Its Role in Water Treatment
Flocculation is a fundamental process in water treatment, crucial for the removal of suspended particles, colloids, and organic matter from water sources. This process leverages the addition of chemical agents, known as flocculants, to aggregate these particles into larger clusters, or flocs, which can then easily be removed through sedimentation or filtration. Understanding the chemistry behind flocculation chemicals is vital for optimizing water treatment processes and ensuring clean water supply.
The Mechanism of Flocculation
Flocculation involves both physical and chemical processes. When flocculants are introduced to water, they interact with suspended particles through a series of steps that include charge neutralization, bridging, and agglomeration. Many suspended particles, such as clay and silt, carry a negative charge, causing them to repel one another. Flocculation chemicals, often based on polymers, possess positive charges that can neutralize this charge, allowing the particles to come together and form larger aggregates.
Once the particles are neutralized, the flocculant molecules can act as a bridge, connecting multiple particles and forming larger, heavier floc structures. The size and density of these flocs significantly enhance their settling properties, making it easier to separate them from water during the treatment process.
Types of Flocculants
There are various types of flocculation chemicals used in water treatment, each with distinct properties and applications. The most common categories include
1. Inorganic Flocculants These include aluminum sulfate (alum), ferric chloride, and polyaluminum chloride. They are widely used due to their effectiveness and low cost. Inorganic flocculants work primarily through charge neutralization and are particularly effective for turbidity removal.
flocculation chemicals
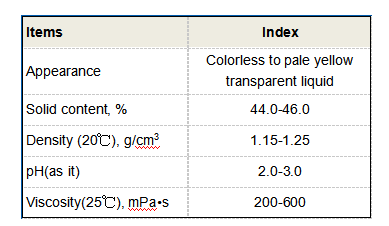
2. Organic Flocculants Synthetic high molecular weight polymers such as polyacrylamides are also popular. They can be cationic, anionic, or nonionic, depending on their charge. Organic flocculants are known for their ability to form larger flocs and can be tailored for specific applications, such as wastewater treatment.
3. Natural Flocculants Derived from natural materials, such as chitosan (from crustacean shells) and starches, these biodegradable options are gaining popularity due to their environmental benefits. They are particularly useful in treating wastewater without introducing harmful chemicals.
Factors Influencing Flocculation
The effectiveness of flocculation depends on several factors, including pH, temperature, concentration of flocculants, and the characteristics of the suspended particles. For instance, the optimal pH for many inorganic flocculants is typically around 6-8, where they can maximize their charge neutralization potential. Temperature can impact the kinetic energy of particles, influencing their collision and aggregation rates.
The choice of flocculant is also crucial. For example, cationic flocculants are often preferred for negatively charged particles, while anionic flocculants may be more effective for positively charged materials. The concentration of the flocculant must be carefully balanced; too little may result in ineffective aggregation, while too much can lead to excess sludge formation and increased treatment costs.
Conclusion
Flocculation is a vital process in the field of water treatment, facilitating the removal of unwanted particles and ensuring clean water. By understanding the chemistry of flocculation chemicals and the factors influencing their effectiveness, industries and municipalities can enhance their water treatment processes, ensuring sustainable and efficient operations. As technology advances and the need for clean water continues to grow, ongoing research into innovative flocculation methods and chemicals will become increasingly important for meeting environmental and public health standards.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





