water treatment flocculation chemicals
Flocculation in Water Treatment Understanding Flocculation Chemicals
Water treatment is an essential process aimed at making water suitable for a specific end-use, either for drinking or for industrial applications. One of the key processes in water treatment is flocculation, which helps in the removal of suspended particles, colloids, and microorganisms. Flocculation involves the agglomeration of particles into larger clusters, known as flocs, which can then be easily removed from the water. At the heart of this process are flocculation chemicals, which play a crucial role in ensuring effective water purification.
What is Flocculation?
Flocculation is a physical-chemical process characterized by the gradual aggregation of small particles into larger ones. This process typically occurs after a preceding step called coagulation, where coagulants are added to destabilize the particles in suspension. Coagulants, often made from aluminum sulfate or ferric chloride, neutralize the electric charges on particles, allowing them to stick together. Once coagulation has taken place, flocculation can occur more efficiently. Gentle mixing helps to further aggregate these destabilized particles into larger flocs, which can be removed through sedimentation or filtration.
Types of Flocculation Chemicals
Flocculation chemicals are mainly classified into two categories coagulants and flocculants.
1. Coagulants These are substances that promote coagulation. Common examples include aluminum sulfate (alum), ferric sulfate, ferric chloride, and polyaluminum chloride (PAC). These chemicals work by neutralizing the surface charges of the particles, which facilitates their aggregation into larger floc structures.
2. Flocculants After coagulation, flocculants are added to enhance the aggregation of particles and improve the settling of flocs. Flocculants are typically long-chain polymers, either synthetic or natural. Common synthetic flocculants include polyacrylamides, while natural options can comprise starches or proteins like chitosan. Flocculants can improve the properties of flocs, making them larger, denser, and easier to remove from water.
Mechanism of Action
water treatment flocculation chemicals
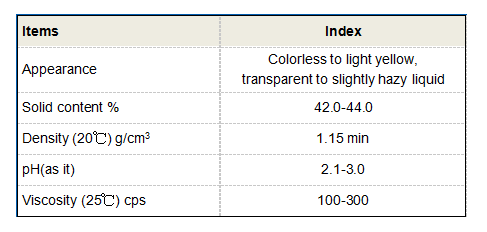
The effectiveness of flocculation chemicals hinges on their ability to bridge the gaps between particles. Coagulants neutralize charges, while flocculants create bridges through polymer chains that connect multiple particles. This mechanism leads to the formation of larger flocs that settle quickly during sedimentation. The size, density, and strength of these flocs significantly affect the efficiency of the water treatment process.
Factors Influencing Flocculation
Several factors influence the success of flocculation, including pH, temperature, mixing intensity, and the dosage of chemicals.
- pH The pH of the water can affect the solubility and charge of the chemicals, impacting their effectiveness. For instance, certain coagulants work best at specific pH ranges. - Temperature The temperature can also affect the kinetics of flocculation. Generally, warmer temperatures enhance the collisions between particles, promoting faster aggregation. - Mixing Intensity Proper mixing is essential. Insufficient mixing may not provide enough energy for particles to collide, while excessive mixing may break down flocs. - Dosage Finding the optimal dosage of coagulants and flocculants is crucial. Under-dosing may lead to ineffective particle removal, while overdosing can result in excess residuals in treated water.
Environmental and Economic Considerations
The use of flocculation chemicals also raises environmental and economic considerations. Certain chemicals may pose risks if not managed properly and can lead to secondary pollution. Therefore, it is essential to conduct thorough risk assessments and consider alternative, more sustainable options, such as natural flocculants from renewable sources.
Cost-effectiveness is another consideration; while some chemicals may offer immediate benefits, long-term costs associated with chemical purchases, handling, and potential environmental compliance should be evaluated.
Conclusion
Flocculation chemicals are vital in the water treatment process, contributing significantly to the removal of impurities and ensuring clean, safe water for consumption and use. By understanding the mechanisms of action and the factors influencing the flocculation process, water treatment facilities can optimize their operations for better efficiency and sustainability, ultimately benefiting both human health and the environment.
-
Pbtc Scale InhibitorPBTC: A Scale Protector for Industrial Water TreatmentNewsAug.05,2025
-
Organic Phosphonate: An Efficient Defender in the Field of Scale InhibitionNewsAug.05,2025
-
Hydrolyzed Polymaleic Anhydride: Green Pioneer in Scale Inhibition FieldNewsAug.05,2025
-
PAPEMP Polyamino Polyether Methylene Phosphonic Acid For SaleNewsAug.05,2025
-
Flocculant Water Treatment: A Pioneer in Purification in the Field of Water TreatmentNewsAug.05,2025
-
Benzyl Isothiazolinone: An Efficient and Broad-Spectrum Antibacterial Protective GuardNewsAug.05,2025





