Understanding Scale Formation and the Role of Corrosion Inhibitors in Chemical Applications
Scale and Corrosion Inhibitor Chemicals An Overview
Scale and corrosion are two prevalent issues in various industrial and domestic systems that rely on water-based processes. These problems not only hinder operational efficiency but can also lead to significant maintenance costs and unexpected downtimes. The effective management of scale and corrosion is critical in industries such as oil and gas, power generation, water treatment, and manufacturing. To combat these challenges, scale and corrosion inhibitor chemicals play a pivotal role.
Scale formation occurs when dissolved minerals in water precipitate and accumulate on surfaces, leading to a thick crust that can obstruct flow, reduce heat transfer, and damage equipment. Common minerals responsible for scaling include calcium carbonate, calcium sulfate, and silica. Corrosion, on the other hand, refers to the gradual degradation of materials, typically metals, due to chemical reactions with their environment, often exacerbated by the presence of oxygen and electrolytes in water.
Inhibitor chemicals are formulated to target both scaling and corrosion, offering a dual approach to maintaining system integrity. These chemicals are designed to alter the properties of scale-forming substances or to create a protective film on metal surfaces. For instance, phosphonates and polyacrylates are widely used as scale inhibitors, effectively preventing the crystallization of scale-forming salts. They work by interfering with the growth of crystal structures, allowing the minerals to remain dissolved in the water.
scale and corrosion inhibitor chemicals
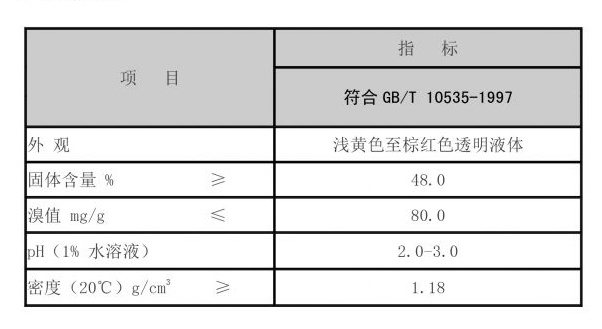
Corrosion inhibitors, such as nitrites, amines, and phosphates, serve to protect surfaces from corrosion. They can do this by passivating the metal surface, which means forming a protective layer that reduces the interaction between the metal and corrosive agents in the environment. The choice of inhibitor depends on factors such as the type of system, the nature of the fluid, and specific environmental conditions.
Employing scale and corrosion inhibitors not only improves system efficiency but also extends the lifespan of the equipment. However, it is essential to monitor the concentration and effectiveness of these chemicals regularly, as overuse can lead to chemical imbalances and environmental concerns.
In conclusion, the use of scale and corrosion inhibitor chemicals is vital for efficient and safe operation within a variety of industries. By understanding the mechanisms through which these inhibitors function, industries can better prevent the detrimental effects of scale and corrosion, ultimately leading to lower maintenance costs and enhanced performance. Proper application and monitoring of these chemicals can ensure a sustainable and efficient approach to managing these critical challenges in water systems.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





