Analysis of Polyaluminium Chloride pH Levels and Their Impact on Water Treatment
Understanding the pH of Polyaluminium Chloride Implications and Applications
Polyaluminium chloride (PAC) is a widely used coagulant in water treatment processes, known for its effectiveness in removing turbidity and other contaminants. Its chemical structure allows it to destabilize colloidal particles, facilitating their agglomeration and subsequent removal from water. One of the critical factors in determining the efficiency of PAC in water treatment is its pH level, which influences both its coagulation performance and the quality of treated water.
What is Polyaluminium Chloride?
Polyaluminium chloride is a polymeric form of aluminium chloride, characterized by its variable composition that can include different hydrolyzed forms of aluminum. Typically produced as a coagulant in liquid or powder forms, PAC features a high charge density, making it particularly effective in various water treatment scenarios, such as drinking water purification, wastewater treatment, and industrial processes. Its ability to bind and aggregate suspended particles is particularly important given the stringent regulations on water quality.
The Importance of pH in Water Treatment
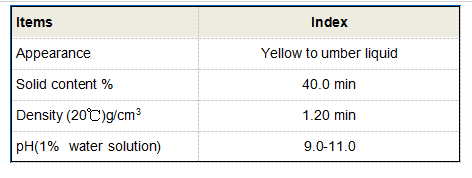
pH is a measure of the acidity or alkalinity of a solution, influencing various chemical equilibria and reactions in water treatment. In the context of PAC, the pH level can significantly affect its coagulation properties. The optimal pH range for PAC usually lies between 6.5 and 8.5, as outside of this range, the coagulation efficiency may decrease.
At lower pH levels (acidic conditions), aluminium species solubility increases, but the charge neutralization capacity may not be optimized for effective particle destabilization. This can lead to inefficient coagulation and increased residual aluminium in treated water. Conversely, in highly alkaline conditions (higher pH levels), aluminium ions can precipitate out as aluminium hydroxide, significantly diminishing the coagulant's effectiveness and potentially leading to higher operational costs due to the need for additional chemical treatments.
The Mechanism of Coagulation
polyaluminium chloride ph
The coagulation process involves several steps charge neutralization, particle agglomeration, and sedimentation. PAC works by neutralizing the negative charges on suspended particles in water, allowing them to aggregate into larger clusters that can settle out more easily. The pH of the water affects both the charge characteristics of these particles and the solubility of the aluminium species present in PAC.
In optimal pH conditions, PAC forms a range of soluble aluminium species, including monomeric and polymeric forms, which effectively interact with colloidal particles. As the pH shifts outside the optimal range, the nature of these interactions changes, affecting both coagulation rates and the final water quality.
Factors Influencing pH in Water Treatment
Several factors can influence the pH in water treatment facilities, including the initial pH of raw water, the presence of organic materials, and the addition of chemicals during treatment. For instance, industrial effluents may have varying pH levels that necessitate careful adjustments before PAC dosage. Moreover, natural organic matter (NOM) can complicate pH management, as it often contains acids that can lower pH and thus influence PAC's efficacy.
Regular monitoring of pH levels is crucial, as it helps operators optimize coagulation conditions. When pH is maintained within an ideal range, the effectiveness of PAC increases, leading to better floc formation and reduced residuals.
Conclusion
In conclusion, understanding the pH of polyaluminium chloride and its influence on coagulation processes is vital for effective water treatment. The efficiency of PAC as a coagulant is closely tied to the pH of the treated water, and optimizing this parameter can yield significant improvements in water quality. As water treatment technologies evolve, continued research and monitoring of pH dynamics in relation to PAC will be essential for enhancing treatment efficiency, ensuring regulatory compliance, and providing safe drinking water. By prioritizing pH management, water treatment facilities can maximize the benefits of PAC, contributing to sustainable water resource management in an increasingly demanding environment.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





