1 2 4 butanetricarboxylic acid1 2 4
Exploring 1,2,4-Butanetricarboxylic Acid Structure, Properties, and Applications
1,2,4-Butanetricarboxylic acid, commonly referred to as citric acid, represents an intriguing compound with significant relevance in various fields, including chemistry, food science, and pharmaceuticals. This organic acid's unique structure, versatile properties, and practical applications make it a topic of considerable interest for researchers and industry professionals alike.
Structure and Characteristics
1,2,4-Butanetricarboxylic acid is characterized by its molecular formula of C₄H₆O₇, which indicates the presence of three carboxylic acid functional groups (-COOH) attached to a butane backbone. The specific positioning of the carboxylic groups on the second and fourth carbon atoms of the butane chain distinguishes it from other carboxylic acids, lending it unique chemical properties.
Its chemical structure can be visualized as a straight-chain aliphatic compound, which is relatively small for a tricarboxylic acid. This compact structure leads to a high degree of solubility in water, making it easy to handle and utilize in various formulations. The acid is known for its colorless, crystalline appearance when in pure form, and it has a distinct sour taste, a characteristic attributed to its acidity.
Chemical Properties
The acidity of 1,2,4-butanetricarboxylic acid stems from its ability to donate protons (H⁺ ions) when dissolved in water. This property is quantified by its pKa values, which indicate that the compound can exist in various ionization states depending on the pH of the environment. As a triprotic acid, it can release three protons sequentially, allowing it to act as a buffering agent in biological and chemical systems.
1 2 4 butanetricarboxylic acid1 2 4
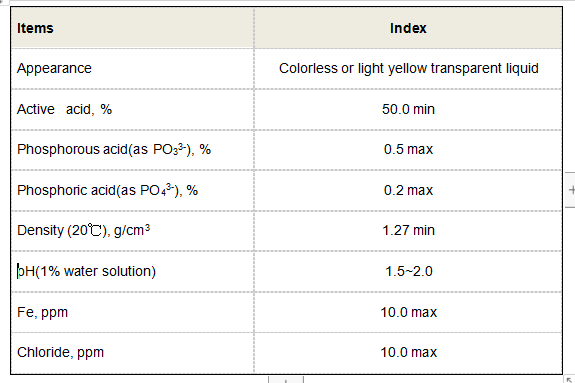
In addition to its acidic nature, 1,2,4-butanetricarboxylic acid is also involved in important biochemical pathways. Notably, it plays a crucial role in the citric acid cycle (Krebs cycle), which is fundamental to energy production in living organisms. This cycle is essential for the metabolism of carbohydrates, fats, and proteins, highlighting the compound's biological significance.
Applications
The diverse applications of 1,2,4-butanetricarboxylic acid span numerous industries. One of its most well-known uses is as a natural preservative in the food and beverage sector. Due to its antimicrobial properties, it inhibits the growth of harmful bacteria and fungi, thus prolonging the shelf life of various food products. Moreover, its ability to enhance flavors makes it a popular ingredient in soft drinks, candies, and preserved foods.
In the cosmetic industry, 1,2,4-butanetricarboxylic acid is often utilized for its chelating properties. It can bind metal ions, which helps to stabilize formulations and prevent deterioration. Additionally, its mild acidity makes it a favorable ingredient in skincare products, where it can act as a pH adjuster and provide exfoliating properties.
The pharmaceutical realm also benefits from this compound. Its derivatives are studied for potential therapeutic applications, including anti-inflammatory and antioxidant effects. The versatility of 1,2,4-butanetricarboxylic acid allows it to be incorporated into various medicinal formulations, enhancing their efficacy and stability.
Conclusion
In summary, 1,2,4-butanetricarboxylic acid is a fascinating compound with a wealth of applications across multiple sectors. Its unique structure and properties contribute to its significance in food preservation, cosmetics, and pharmaceuticals. As research continues to uncover new potentials for this remarkable organic acid, its importance is likely to grow, highlighting the necessity of further exploration into its diverse functionalities. Understanding compounds like 1,2,4-butanetricarboxylic acid not only advances scientific knowledge but also enables innovation across various industries.
-
Pbtc Scale InhibitorPBTC: A Scale Protector for Industrial Water TreatmentNewsAug.05,2025
-
Organic Phosphonate: An Efficient Defender in the Field of Scale InhibitionNewsAug.05,2025
-
Hydrolyzed Polymaleic Anhydride: Green Pioneer in Scale Inhibition FieldNewsAug.05,2025
-
PAPEMP Polyamino Polyether Methylene Phosphonic Acid For SaleNewsAug.05,2025
-
Flocculant Water Treatment: A Pioneer in Purification in the Field of Water TreatmentNewsAug.05,2025
-
Benzyl Isothiazolinone: An Efficient and Broad-Spectrum Antibacterial Protective GuardNewsAug.05,2025





