butane 1 2 4 tricarboxylic acid
Exploring Butane-1,2,4-tricarboxylic Acid Structure, Properties, and Applications
Butane-1,2,4-tricarboxylic acid, also known as mesaconic acid, is an interesting compound that serves as a key intermediate in organic synthesis and biochemical pathways. Its structure features a butane chain with three carboxylic acid groups (-COOH) situated at each end and in the middle, specifically at positions 1, 2, and 4. This unique arrangement of functional groups presents a diverse array of physical and chemical properties which make butane-1,2,4-tricarboxylic acid notable in various scientific fields.
Structure and Properties
The molecular formula of butane-1,2,4-tricarboxylic acid is C₅H₈O₆, which indicates the presence of five carbon atoms, eight hydrogen atoms, and six oxygen atoms. The compound can be visualized as a straight-chain alkane with its carboxylic functional groups providing acidity and reactivity. The positioning of these groups contributes to the compound's acidity, making it a triacidic molecule.
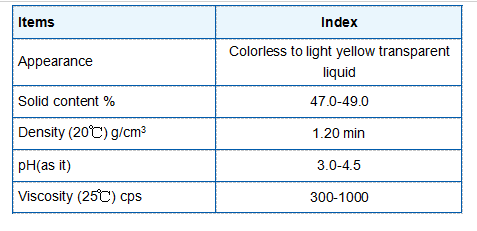
With a molecular weight of approximately 176.12 g/mol, butane-1,2,4-tricarboxylic acid has a melting point around 153 °C and a boiling point that exceeds 300 °C. Its solubility in water is a significant property, as the polar nature of the carboxylic acid groups allows for hydrogen bonding with water molecules, facilitating its use in various aqueous chemical reactions.
Synthesis Pathways
Butane-1,2,4-tricarboxylic acid can be synthesized through several methods, often involving the oxidation of simpler hydrocarbons or the biological fermentation of organic substrates. In laboratory settings, it can be prepared via the multistep synthesis starting from alkenes or alkynes, allowing for controlled formation of the desired functional groups.
butane 1 2 4 tricarboxylic acid
Additionally, it plays a role in several biochemical pathways; for instance, it is an intermediate in the metabolism of certain amino acids and in the citric acid cycle through the formation of aconitic acid. Understanding these pathways can provide insights into its importance in biological systems.
Applications
The multifaceted nature of butane-1,2,4-tricarboxylic acid leads to several practical applications, particularly in the fields of organic chemistry, biochemistry, and materials science. Its primary utility lies in the synthesis of pharmaceuticals, agrochemicals, and polymers. The compound's carboxylic acid groups render it reactive towards alcohols, amines, and various nucleophiles, which can be harnessed to create a variety of derivatives with tailored properties.
In biochemistry, butane-1,2,4-tricarboxylic acid's role as a metabolic intermediate emphasizes its significance in biochemical reactions and cellular processes. Studies investigating its metabolic pathways may uncover further applications in biomedical research, particularly in understanding metabolic disorders or developing novel therapeutic agents.
Moreover, its potential use in the production of biodegradable polymers highlights the compound's role in sustainable materials science. As the world pivots towards environmentally friendly alternatives to traditional plastics, compounds like butane-1,2,4-tricarboxylic acid present an exciting area of research. By acting as a building block for biopolymers, it contributes to reducing reliance on fossil fuels while promoting more sustainable manufacturing practices.
Conclusion
Butane-1,2,4-tricarboxylic acid is a compound of significant chemical interest, due to its unique structural features and versatile applications. From its role as a biochemical intermediate to its potential in sustainable materials production, the compound epitomizes the intersection of chemistry and practical application. Continued research into butane-1,2,4-tricarboxylic acid will undoubtedly unveil new insights, enabling advancements not only in organic synthesis but also in various scientific endeavors that address contemporary challenges in health, environment, and technology. As we explore the capabilities of this compound, we uncover deeper layers of its significance in both science and industry.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





