1 2 4 butanetricarboxylic acid1 2 4
Exploring the Properties and Applications of 1,2,4-Butanetricarboxylic Acid
1,2,4-Butanetricarboxylic acid (also known as citric acid and by its chemical formula C7H10O7) is a versatile organic compound with significant applications in various fields, most notably in the food and pharmaceutical industries. This article delves into its chemical properties, synthesis, and practical uses, highlighting why this compound is vital in both scientific research and everyday applications.
Chemical Properties
1,2,4-Butanetricarboxylic acid features three carboxyl (-COOH) functional groups, making it a tricarboxylic acid. The presence of these functional groups contributes to its acidity, allowing it to act as a weak acid in aqueous solutions. Its molecular structure consists of a butane backbone to which three carboxylic acid groups are attached. This structural configuration imparts not only acidity but also a degree of versatility in reacting with various molecules.
The acid has a melting point of approximately 153-156 °C and is soluble in water, ethanol, and other polar solvents. Its ability to chelate metal ions enhances its utility in various chemical processes, particularly in agriculture and the food industry where it helps stabilize certain compounds.
Synthesis
The synthesis of 1,2,4-butanetricarboxylic acid can be achieved through various methods. One common approach involves the fermentation of carbohydrates, where certain strains of fungi or bacteria produce this compound as a metabolic by-product. Citric acid, a close relative, is frequently produced commercially via the fermentation of sugars, utilizing the mold Aspergillus niger.
Alternatively, it can also be synthesized through chemical reactions involving suitable precursors. For example, a multistep synthesis might begin with the carboxylation of butane derivatives, followed by selective oxidations to introduce the necessary functional groups.
Applications
Food Industry
1 2 4 butanetricarboxylic acid1 2 4
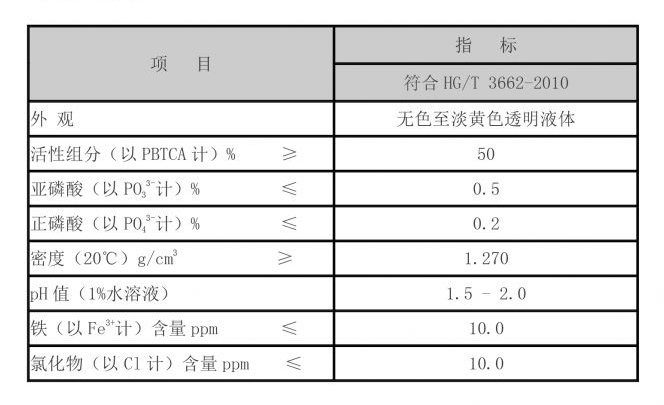
1,2,4-Butanetricarboxylic acid is most widely recognized for its role as a food additive. As a natural preservative and flavoring agent, it enhances the taste of various food products while also prolonging their shelf life. It is commonly used in citrus-flavored beverages, candies, and processed foods. Its chelating property enables it to bind with metal ions, which can improve the stability and texture of food products.
Moreover, its sour flavor profile allows it to serve as a flavor enhancer, masking undesirable tastes in some formulations. The acid is often utilized in the production of baking powders, acting as a leavening agent by releasing carbon dioxide when combined with baking soda.
Pharmaceuticals
In the pharmaceutical field, 1,2,4-butanetricarboxylic acid is employed in the formulation of various medications. Its ability to act as a buffering agent helps maintain the pH balance of drug solutions, ensuring the stability of active ingredients. In addition, its chelating properties are utilized in drug delivery systems to improve the solubility and bioavailability of certain medications.
Research has increasingly focused on the potential therapeutic uses of this compound, including its antioxidant properties. Antioxidants are vital in protecting cells from oxidative stress, which is linked to various chronic conditions, including cardiovascular diseases and cancer. Consequently, 1,2,4-butanetricarboxylic acid is being investigated for its potential role in health supplements aimed at enhancing overall wellness.
Agriculture
In agriculture, 1,2,4-butanetricarboxylic acid finds applications as a chelating agent in fertilizers, which helps improve the bioavailability of essential nutrients for plants. By binding to metal ions, it ensures that these nutrients remain soluble and accessible for plant uptake. This is particularly important in soils with high pH levels, where certain nutrients become insoluble and thus unavailable to plants.
Conclusion
1,2,4-butanetricarboxylic acid is a multifaceted compound with significant importance across diverse industries, particularly in food, pharmaceuticals, and agriculture. Its unique chemical properties, including its acidity and ability to chelate metal ions, warrant its continued study and application. As research progresses, this compound could unveil even more potential uses, reinforcing its status as a vital component in modern industry and science. The ongoing exploration of its benefits ensures that 1,2,4-butanetricarboxylic acid will remain a key player in a variety of essential applications for years to come.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





