butane 1 2 4 tricarboxylic acid
Butane-1,2,4-Tricarboxylic Acid A Comprehensive Overview
Butane-1,2,4-tricarboxylic acid, also known as tricarballylic acid, is a significant organic compound with increased relevance in various fields, including organic chemistry, biochemistry, and material science. As a multifunctional dicarboxylic acid, it comprises a four-carbon chain with three carboxylic acid functional groups located at the first, second, and fourth positions. This unique structure endows butane-1,2,4-tricarboxylic acid with distinctive properties and versatile applications.
Chemical Structure and Properties
The molecular formula of butane-1,2,4-tricarboxylic acid is C₄H₆O₆, featuring three −COOH groups, which significantly influence its acidic characteristics and solubility. The compound can exist in different forms, mainly as solid and liquid states, depending on temperature and concentration. Butane-1,2,4-tricarboxylic acid can demonstrate varying degrees of acidity, owing to the presence of multiple carboxylic acid groups, making it a useful acid in various chemical reactions.
One of its most intriguing properties is its ability to form stable complexes with metal ions, resulting in a wide range of applications, particularly in catalysis and coordination chemistry. In addition, butane-1,2,4-tricarboxylic acid has a higher molecular weight than many other simple organic acids, leading to different physical properties such as melting and boiling points, making it essential for specific industrial processes.
Synthesis and Production
Butane-1,2,4-tricarboxylic acid can be synthesized through various methods, including oxidation of hydrocarbons and fermentation processes utilizing specific microorganisms. One common synthetic route involves the oxidation of maleic acid or fumaric acid in the presence of suitable catalytic systems. Advances in biotechnology have also paved the way for producing the compound through microbial fermentation, which not only provides a sustainable approach but also reduces the reliance on fossil fuels.
butane 1 2 4 tricarboxylic acid
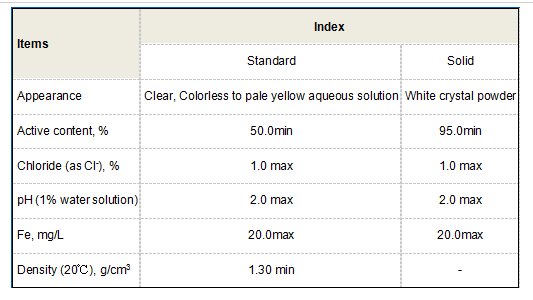
The choice of synthesis method can influence the purity and yield of the final product. Ensuring high purity is essential for its application in food, pharmaceuticals, and cosmetic industries, where contaminants can pose health risks. Therefore, optimizing production methods and understanding reaction parameters is critical for obtaining desirable quality.
Applications
The applications of butane-1,2,4-tricarboxylic acid span across several industries. In the pharmaceutical sector, it is employed as an intermediate in the synthesis of various active pharmaceutical ingredients. Its ability to form esters and amides enhances its utility in producing complex organic molecules.
Moreover, butane-1,2,4-tricarboxylic acid serves as a chelating agent in coordination chemistry. The presence of multiple carboxylic acid groups allows it to bind multiple metal ions, making it valuable in applications involving heavy metal remediation and catalysis. In this regard, it is utilized in developing catalysts for biodiesel production and environmental cleaning techniques.
In the food industry, butane-1,2,4-tricarboxylic acid acts as a flavoring agent and food preservative. Its acidity can enhance flavor profiles and improve the stability of food products, extending shelf life. Moreover, its capability to act as a pH regulator is beneficial in various food formulations.
Conclusion
In summary, butane-1,2,4-tricarboxylic acid is a remarkable compound with multifaceted properties and applications that span various industries. Its unique structure, characterized by a backbone of four carbon atoms and three carboxylic acid groups, endows it with distinct chemical behavior and versatility. As research continues to uncover new methods of synthesis and potential applications, butane-1,2,4-tricarboxylic acid is poised to play an increasingly important role in advancing chemical science, sustainable practices, and innovative industrial processes. Understanding its characteristics and applications not only expands current scientific knowledge but also enhances its utility in practical applications, thereby contributing to various fields, including pharmaceuticals, food technology, and environmental science.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





