Exploring the Role of Flocculant Chemicals in Effective Water Treatment Processes and Solutions
Flocculant Chemicals for Water Treatment
Water treatment is a crucial process in ensuring that water is safe for consumption and various industrial uses. Among the key agents used in water treatment are flocculant chemicals, which play an essential role in clarifying water and removing suspended particles. This article will explore the types, mechanisms, and applications of flocculant chemicals in water treatment processes.
What are Flocculants?
Flocculants are chemical substances that promote the clumping of fine particles into a floc, which can then be easily removed from the water. They are typically used in processes such as sedimentation, where suspended solids settle out of the liquid, and in filtration, where the flocs can be screened out. The effectiveness of flocculants is governed by their molecular properties, including charge density, molecular weight, and structure.
Types of Flocculants
Flocculants can be classified into three main categories natural, synthetic organic, and inorganic flocculants.
1. Natural Flocculants These are derived from plant or animal sources. Common examples include starch, guar gum, and chitosan. Natural flocculants are often preferred because they are biodegradable and environmentally friendly, making them suitable for applications in drinking water treatment as well as in food processing.
2. Synthetic Organic Flocculants These are man-made polymers, usually based on acrylamide, that have been modified to enhance their flocculating properties. Synthetic flocculants can be more effective than their natural counterparts in certain scenarios due to their tailored molecular structures. Common examples include polyacrylamide (PAM) and poly(diallyldimethylammonium chloride) (dADMAC). However, concerns about the potential toxicity of some synthetic flocculants have led to careful regulation.
3. Inorganic Flocculants Common inorganic flocculants include aluminum sulfate (alum) and ferric chloride. These chemicals work by neutralizing the charges on suspended particles, allowing them to aggregate and settle more efficiently. Inorganic flocculants are widely used due to their cost-effectiveness and high efficiency in a variety of water treatment scenarios.
flocculant chemicals for water treatment
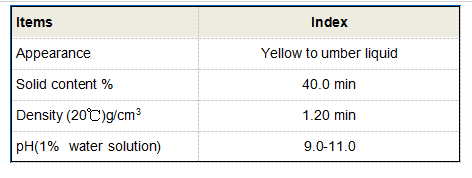
Mechanism of Action
The flocculation process typically begins with the neutralization of the electric charges on suspended particles. Most fine particles in water carry a negative charge, causing them to repel each other. When flocculant chemicals are introduced, they neutralize the particles' charge, allowing them to come closer together. As these particles collide, they form larger aggregates known as flocs, which can then be removed through sedimentation or filtration.
The effectiveness of this process can be influenced by various factors, including pH, temperature, and the concentration of the flocculant used. Additionally, the mixing energy applied during the treatment process can impact the size and strength of the resulting flocs.
Applications in Water Treatment
Flocculants are used in a wide range of water treatment applications. In municipal water treatment plants, they are critical for producing potable water by removing turbidity and pathogenic microorganisms. In industrial settings, flocculants help to treat wastewater by facilitating the removal of contaminants before the water is discharged back into the environment.
Furthermore, flocculants are also employed in the mining and mineral processing industries, where they aid in separating valuable minerals from ores, thereby enhancing resource recovery.
Conclusion
Flocculant chemicals are indispensable in modern water treatment processes. Their ability to effectively aggregate suspended particles leads to clearer, safer water for both human consumption and industrial use. As regulations around water quality become stricter and environmental concerns continue to grow, the development and application of eco-friendly flocculants remain an area of significant interest and innovation. Ultimately, the effective use of flocculants contributes to a sustainable approach to managing our most precious resource water.
-
Pbtc Scale InhibitorPBTC: A Scale Protector for Industrial Water TreatmentNewsAug.05,2025
-
Organic Phosphonate: An Efficient Defender in the Field of Scale InhibitionNewsAug.05,2025
-
Hydrolyzed Polymaleic Anhydride: Green Pioneer in Scale Inhibition FieldNewsAug.05,2025
-
PAPEMP Polyamino Polyether Methylene Phosphonic Acid For SaleNewsAug.05,2025
-
Flocculant Water Treatment: A Pioneer in Purification in the Field of Water TreatmentNewsAug.05,2025
-
Benzyl Isothiazolinone: An Efficient and Broad-Spectrum Antibacterial Protective GuardNewsAug.05,2025





