pbtc tricarboxylic acid
The Role of PBTC in the Tricarboxylic Acid Cycle
The tricarboxylic acid (TCA) cycle, also known as the Krebs cycle or citric acid cycle, is a fundamental metabolic pathway that plays a pivotal role in cellular respiration. It serves as the primary mechanism through which organisms extract energy from nutrients. Among the various components involved in this cycle, PBTC (Phosphono-Butyric Tricarboxylic Acid) has emerged as a significant compound of interest due to its unique properties and potential applications.
The Role of PBTC in the Tricarboxylic Acid Cycle
In the context of the TCA cycle, PBTC's relevance extends beyond its chelation properties. The TCA cycle is integral to the metabolic processes of aerobic organisms, facilitating the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. Through a series of enzymatic reactions, the cycle produces key metabolites such as NADH and FADH2, which are essential for the generation of ATP via the electron transport chain.
pbtc tricarboxylic acid
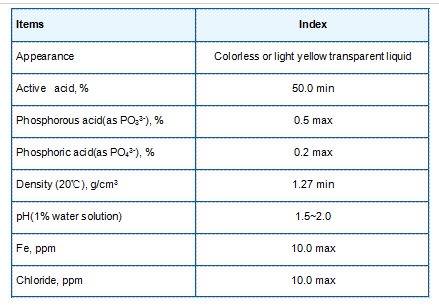
Research has indicated that the introduction of PBTC into biological systems might influence the efficiency of metabolic pathways, including the TCA cycle. By modulating the availability of metal ions, PBTC could potentially enhance enzymatic reactions that rely on these cofactors. For instance, certain enzymes within the TCA cycle, such as aconitase and alpha-ketoglutarate dehydrogenase, require metal ions like iron and magnesium for optimal activity. Therefore, the strategic use of PBTC could improve metabolic efficiency, promoting higher energy yields from substrates.
Furthermore, PBTC's properties as a phosphonate compound highlight its potential as a biochemical tool. Phosphonates are known for their stability and resistance to biological degradation, which makes them suitable for therapeutic applications. Studies have suggested that PBTC and its analogs could be developed into novel therapeutic agents for diseases associated with metabolic dysfunctions, including diabetes and obesity. By targeting the TCA cycle, these compounds may restore metabolic balance and improve cellular energy homeostasis.
In addition to its implications in medicine, PBTC can be leveraged in agricultural sciences. The chelating properties of PBTC can improve nutrient availability in soils, enhancing plant growth and productivity. By preventing the fixation of essential metal nutrients, PBTC ensures that plants can efficiently uptake necessary ions, promoting robust agricultural outputs. This application is particularly crucial in regions with nutrient-deficient soils, as it provides a sustainable solution to improve crop yields.
In conclusion, the role of PBTC in the tricarboxylic acid cycle and beyond is multifaceted. Its unique structural properties not only make it a powerful chelating agent but also a potential enhancer of metabolic pathways and therapeutic agent. As research continues to uncover the biochemical mechanisms at play, PBTC stands out as a compound of significant interest, with implications that stretch across various fields, including medicine, agriculture, and industrial applications. The ongoing exploration of PBTC will likely yield valuable insights that could lead to innovative solutions in enhancing energy metabolism and nutrient management.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





