Feb . 10, 2025 09:41
Back to list
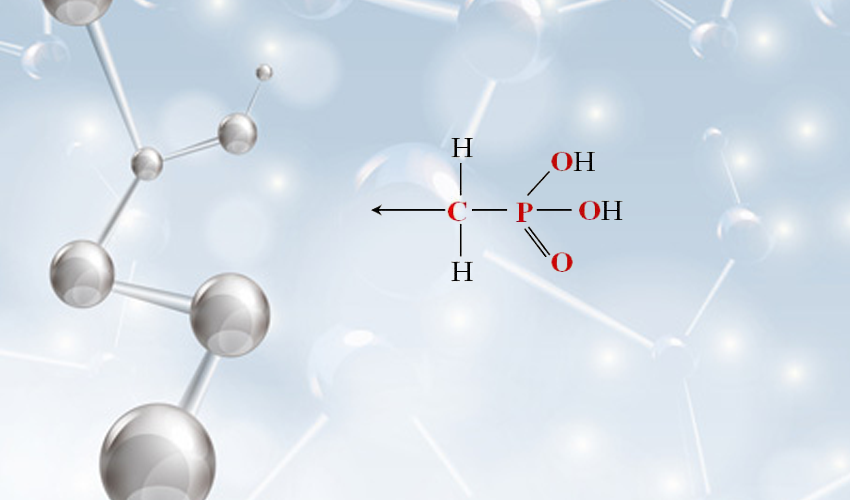
phosphorothioate
Phosphorothioates, a unique class of compounds, are chemically modified backbones of nucleotides where one non-bridging oxygen in the phosphate backbone is replaced by sulfur. This seemingly minor substitution unleashes a myriad of properties that have radically transformed research and therapeutic landscapes. Their introduction into oligonucleotides enhances resistance to nucleases and improves binding affinity, presenting unparalleled opportunities in therapeutic applications and beyond.
Moreover, regulatory bodies have increasingly recognized the importance of phosphorothioates, backing their inclusion in groundbreaking therapies with accelerated approval processes, thereby enriching the competitive landscape. Stakeholders focusing on intellectual property must seize the opportunity to safeguard their innovations around phosphorothioate chemistry and delivery systems, as this domain burgeons with commercial potential. For educational institutions and labs seeking to ride the phosphorothioate wave, fostering strategic partnerships between academia, research institutes, and biotech companies becomes essential. These alliances can expedite the exchange of insights and accelerate the translation of phosphorothioate-centered research into market-ready applications. Ultimately, the trustworthiness of phosphorothioate products resides in ongoing research validations, ecosystem collaborations, and regulatory endorsements. As experts, emphasizing a robust understanding of phosphorothioate's biochemical nuances while encouraging ethical use will be paramount in advancing both the scientific community's goals and market demands. In conclusion, phosphorothioates embody the forefront of innovation in nucleic acid therapeutics, bearing a transformative potential that could redefine therapeutic efficacy and accessibility. By navigating the intricate balance of chemical innovation, delivery optimization, and rigorous validation, we expand the horizons of what phosphorothioate-enhanced products can achieve in healthcare and beyond. The journey promises not only profound implications for human health but a genuine augmentation of our molecular toolkit.

Moreover, regulatory bodies have increasingly recognized the importance of phosphorothioates, backing their inclusion in groundbreaking therapies with accelerated approval processes, thereby enriching the competitive landscape. Stakeholders focusing on intellectual property must seize the opportunity to safeguard their innovations around phosphorothioate chemistry and delivery systems, as this domain burgeons with commercial potential. For educational institutions and labs seeking to ride the phosphorothioate wave, fostering strategic partnerships between academia, research institutes, and biotech companies becomes essential. These alliances can expedite the exchange of insights and accelerate the translation of phosphorothioate-centered research into market-ready applications. Ultimately, the trustworthiness of phosphorothioate products resides in ongoing research validations, ecosystem collaborations, and regulatory endorsements. As experts, emphasizing a robust understanding of phosphorothioate's biochemical nuances while encouraging ethical use will be paramount in advancing both the scientific community's goals and market demands. In conclusion, phosphorothioates embody the forefront of innovation in nucleic acid therapeutics, bearing a transformative potential that could redefine therapeutic efficacy and accessibility. By navigating the intricate balance of chemical innovation, delivery optimization, and rigorous validation, we expand the horizons of what phosphorothioate-enhanced products can achieve in healthcare and beyond. The journey promises not only profound implications for human health but a genuine augmentation of our molecular toolkit.
Share
Next:
Latest news
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





