polyaspartic acid structure
The Structure and Significance of Polyaspartic Acid
Polyaspartic acid, often abbreviated as PAS or PA, is a naturally occurring polymer derived from the amino acid aspartic acid. With its unique structural characteristics, polyaspartic acid has garnered significant attention in various fields, including biochemistry, materials science, and medicine. Understanding its structure is crucial to appreciating its diverse applications and benefits.
Chemical Structure of Polyaspartic Acid
Polyaspartic acid is a polyamino acid, consisting of repeating units of aspartic acid. The monomeric unit of polyaspartic acid features a backbone made of carbon atoms that are linked by peptide bonds, forming a linear polymer chain. The structure of polyaspartic acid is characterized by the presence of two functional groups associated with each repeating unit a carboxylic acid group (-COOH) and an amine group (-NH2).
This arrangement results in a polymer that is hydrophilic, enabling it to interact favorably with water and other polar substances. The carboxylic groups can ionize in solution, generating a negative charge that contributes to the polymer's solubility and reactivity. This unique feature of polyaspartic acid allows it to form various derivatives and complexes, which enhances its functionality in different applications.
Synthesis of Polyaspartic Acid
The synthesis of polyaspartic acid can be achieved through several methods, the most common being the polymerization of aspartic acid. This process typically involves condensation reactions, where water is eliminated as the polymer chains form. Alternatively, polyaspartic acid can be produced through more advanced chemical techniques such as ring-opening polymerization or enzymatic processes, which can yield tailored polymers with specific molecular weights and properties.
Applications of Polyaspartic Acid
The versatile structure of polyaspartic acid lends itself to a wide array of applications. In the biomedical field, it is explored for drug delivery systems due to its biocompatibility and biodegradability. The presence of the carboxylic acids allows for the conjugation of various therapeutic agents, making it an attractive candidate for targeted drug delivery.
polyaspartic acid structure
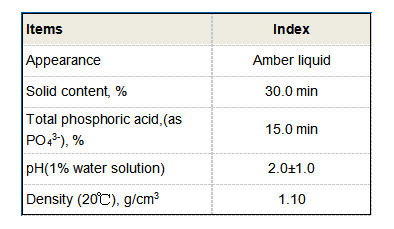
In materials science, polyaspartic acid is utilized in the production of coatings and adhesives. Its excellent adhesion properties and resistance to harsh environmental conditions make it suitable for use in construction and automotive industries. Polyaspartic acid-based coatings have also been developed as alternatives to conventional polyurethanes, offering benefits such as faster curing times and lower volatile organic compounds (VOCs).
Furthermore, polyaspartic acid serves as a chelating agent in water treatment processes. Its ability to bind with metal ions helps prevent scale formation and corrosion in various industrial applications, contributing to more sustainable practices.
Environmental Implications
One of the most significant advantages of polyaspartic acid is its environmentally friendly nature. Unlike many synthetic polymers derived from petrochemicals, polyaspartic acid can be synthesized from renewable resources. Additionally, its biodegradability ensures that it breaks down into non-toxic byproducts, minimizing environmental impact. As industries move toward more sustainable practices, polyaspartic acid represents a promising alternative to traditional materials.
Challenges and Future Perspectives
Despite its numerous advantages, challenges remain in the commercial application of polyaspartic acid. The cost of production and scalability can be limiting factors. Researchers are actively exploring more efficient synthesis methods and ways to integrate polyaspartic acid into various products.
Looking forward, the future of polyaspartic acid appears promising. Ongoing research aims to enhance its properties through copolymerization and functionalization, opening new avenues for application. The potential to develop smart materials that respond to environmental stimuli or to further improve drug delivery systems could revolutionize fields like medicine and material science.
Conclusion
In conclusion, polyaspartic acid is a versatile and significant polymer characterized by its unique structure and functional properties. Its applications across various fields underscore its importance, making it a subject of continued research and development. As the demand for sustainable and innovative materials increases, polyaspartic acid stands as a leading candidate for a greener future.
-
Dodecyldimethylbenzylammonium Chloride: High-Purity DisinfectantNewsAug.30,2025
-
2-Phosphonobutane-1,2,4-Tricarboxylic Acid: Scale & CorrosionNewsAug.29,2025
-
Premium Isothiazolinones | Broad-Spectrum Biocidal SolutionsNewsAug.28,2025
-
LK-319 Special Scale And Corrosion Inhibitor For Steel Plants: Advanced Solutions for Industrial Water SystemsNewsAug.22,2025
-
Flocculant Water Treatment: Essential Chemical Solutions for Purification ProcessesNewsAug.22,2025
-
Isothiazolinones: Versatile Microbial Control Agents for Industrial and Consumer ApplicationsNewsAug.22,2025





