Synthesis and Applications of Polycarboxylic Acids in Modern Chemistry
Polycarboxylic acids are a fascinating class of compounds that play significant roles in both chemistry and industrial applications. Characterized by the presence of multiple carboxyl (-COOH) functional groups, these acids exhibit unique properties that make them valuable in a variety of fields, including organic synthesis, materials science, and pharmaceuticals.
One of the most prominent examples of polycarboxylic acids is citric acid, which contains three carboxyl groups and is widely found in citrus fruits. Citric acid is not only important in culinary applications as a flavoring agent and preservative, but it also serves as a critical intermediate in the citric acid cycle, a vital metabolic pathway that occurs in all aerobic organisms. This highlights the importance of polycarboxylic acids in biological systems, where they contribute to energy production and metabolic regulation.
In addition to citric acid, other well-known polycarboxylic acids include malic acid, tartaric acid, and oxalic acid. Each of these acids has distinct properties and uses. For instance, tartaric acid is often used in baking and winemaking, while oxalic acid serves as a cleaning agent and is utilized in the textile industry. The versatility of polycarboxylic acids extends beyond these applications, as they are also utilized in manufacturing biodegradable polymers and surfactants.
The ability of polycarboxylic acids to form complexes with metal ions further enhances their utility. This chelating property allows them to be effective in various applications, such as water treatment, where they can help remove heavy metals. Moreover, in agriculture, polycarboxylic acids are used as soil conditioners and chelating agents to improve nutrient availability for plants. This aspect is particularly crucial for sustainable agriculture practices, as it promotes the use of environmentally friendly materials.
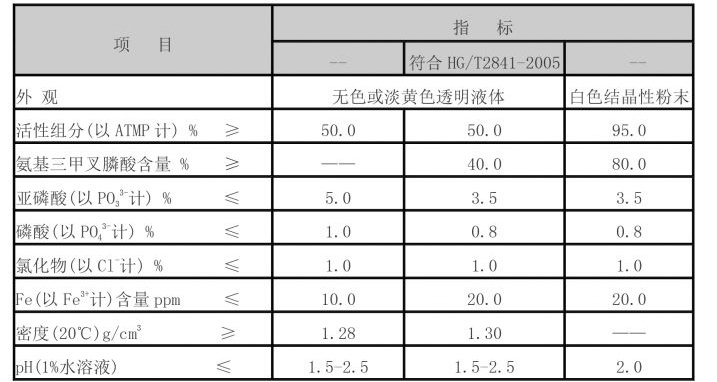
polycarboxylic
In the realm of materials science, polycarboxylic acids are crucial for the synthesis of polymers. For example, they are used in the production of polycarboxylate superplasticizers, which are additives that enhance the workability of concrete and are essential for modern construction. By reducing the amount of water needed for concrete mixtures, these superplasticizers contribute to the development of stronger, more durable structures. Their ability to disperse cement particles not only improves the fluidity of the mixture but also reduces the overall environmental impact of concrete production.
As researchers continue to explore the properties and applications of polycarboxylic acids, innovations continue to emerge. Advances in green chemistry aim to utilize these acids in more eco-friendly processes, such as the development of biodegradable materials that can reduce plastic waste. The potential to modify polycarboxylic acids to enhance their functions opens avenues for novel applications, particularly in drug delivery systems and specialized coatings.
In conclusion, polycarboxylic acids are an essential component of various scientific and industrial sectors. Their unique chemical properties and versatile applications make them invaluable in both traditional and modern contexts. As ongoing research uncovers new ways to harness the potential of these compounds, the importance of polycarboxylic acids in promoting sustainability and innovation will undoubtedly increase, paving the way for a greener future.
-
lk-319-special-scale-and-corrosion-inhibitor-for-steel-plants-advanced-solutions-for-industrial-water-systemsNewsAug.22,2025
-
flocculant-water-treatment-essential-chemical-solutions-for-purification-processesNewsAug.22,2025
-
isothiazolinones-versatile-microbial-control-agents-for-industrial-and-consumer-applicationsNewsAug.22,2025
-
scale-inhibitor-key-solutions-for-water-system-scale-preventionNewsAug.22,2025
-
organophosphonates-versatile-scale-inhibitors-for-industrial-water-systemsNewsAug.22,2025
-
scale-and-corrosion-inhibitor-essential-chemical-solutions-for-water-system-maintenanceNewsAug.22,2025





