Optimization of Polyaluminium Chloride pH for Enhanced Water Treatment Efficiency
Understanding the pH of Polyaluminium Chloride Implications and Applications
Polyaluminium chloride (PAC) is a widely used coagulant in water treatment processes. Its use is prevalent in drinking water purification, wastewater treatment, and various industrial applications. One critical aspect that influences the effectiveness of PAC is its pH level, which plays a significant role in determining the coagulant's performance and the overall quality of treated water.
PAC is a complex chemical compound that exhibits varying properties based on its aluminum content and the degree of polymerization. When PAC is dissolved in water, it hydrolyzes to form aluminum hydroxide, which is essential for aggregation and removal of suspended particles, organic matter, and contaminants. The pH of the solution can significantly affect this hydrolysis process.
Understanding the pH of Polyaluminium Chloride Implications and Applications
Conversely, at higher pH levels, the aluminum complexes can precipitate too quickly, leading to insufficient contact time with suspended particles and inadequate coagulation. Therefore, maintaining an appropriate pH is crucial for maximizing the coagulant’s efficacy in treating water.
polyaluminium chloride ph
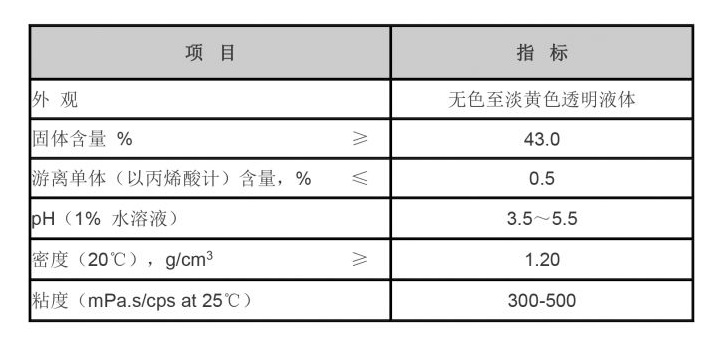
The pH of the water being treated also affects the characteristics of the PAC itself. For instance, in alkaline conditions, PAC may form different hydroxylaluminum species which can impact the size and strength of the flocs produced. Stronger and more stable flocs are vital for effective sedimentation and removal of pollutants.
In practical applications, operators must routinely monitor and adjust the pH of both the PAC solution and the water being treated to ensure optimal performance. This can often be achieved through the addition of pH adjusting agents such as sulfuric acid or sodium hydroxide depending on whether the pH needs to be lowered or raised.
The implications of pH on PAC performance are critical, not only for operational efficiency but also for compliance with regulatory standards concerning residual aluminum levels in drinking water. Understanding and controlling the pH can enhance the coagulation process, reduce chemical usage, and improve the quality of water treatment outcomes.
In conclusion, the pH of polyaluminium chloride is a pivotal factor influencing its coagulative properties. By recognizing the intricate relationship between PAC and pH, water treatment facilities can optimize their processes, yielding cleaner water while adhering to safety standards. Regular monitoring and adjustments of pH are essential practices that should be integrated into the water treatment protocols to harness the full potential of PAC as a coagulant.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





