Isothiazolinone and Its Applications in Modern Chemical Industries and Consumer Products
Understanding Methylisothiazolinone Applications and Concerns
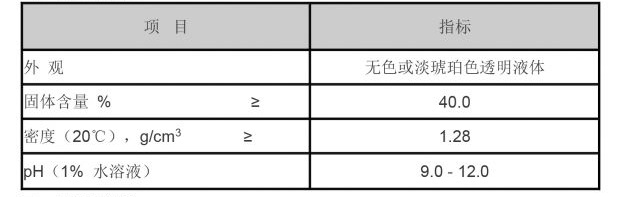
Methylisothiazolinone (MIT) is a synthetic compound known for its effectiveness as a preservative and biocide, particularly in cosmetic and personal care products. As a member of the isothiazolinone family, MIT was developed in the 1970s to address the growing need for efficient antimicrobial agents. Its unique chemical structure allows it to inhibit the growth of bacteria, fungi, and algae, making it a popular choice in various industries.
Applications of Methylisothiazolinone
MIT is commonly found in a wide array of products, including shampoos, conditioners, lotions, wipes, and household cleaners. One of its primary roles is to prevent microbial contamination, extending the shelf life of products and ensuring their safety for consumers. Because of its potency at low concentrations, it is an attractive solution for manufacturers aiming to maintain product integrity without compromising performance.
In the cosmetics industry, MIT is often combined with another preservative, methylchloroisothiazolinone (MCI), in formulations that require robust antimicrobial protection. This combination is effective against a broad spectrum of microorganisms, making it particularly useful in water-based products where bacteria can thrive.
Beyond cosmetics, MIT is used in industrial applications, such as paint and coating formulations. Its fungicidal properties help in preventing the growth of mold and mildew, which can compromise the quality and durability of these products. Additionally, MIT is employed in cooling systems, paper manufacturing, and oil and gas applications, highlighting its versatility as a preservative across various industries.
Safety and Environmental Concerns
me isothiazolinone
While MIT has proven to be a valuable preservative, growing concerns regarding its safety profile have emerged over the years. The compound has been linked to allergic reactions, particularly in the form of contact dermatitis. These reactions have prompted regulatory agencies and advocacy groups to scrutinize its use, especially in products designed for leave-on applications, such as lotions and creams.
In 2013, the European Commission’s Scientific Committee on Consumer Safety (SCCS) assessed the safety of MIT and recommended limiting its concentration in cosmetic formulations. The committee concluded that while MIT is safe at low concentrations in rinse-off products, the risks associated with leave-on products could not be overlooked. Following this, many manufacturers began reformulating their products to reduce or eliminate MIT to mitigate potential health risks.
Moreover, environmental concerns have been raised regarding the persistence of MIT in aquatic ecosystems. Studies suggest that the compound may be toxic to aquatic organisms, leading to caution in its use in products that may end up in waterways.
Future Directions
As awareness grows regarding the potential health and environmental risks associated with certain preservatives, the industry is moving towards developing safer alternatives. Many companies are exploring natural preservatives derived from plant extracts or other sustainable sources. These alternatives not only meet safety regulations but also appeal to consumers looking for greener, eco-friendly products.
In conclusion, while methylisothiazolinone has played a significant role in preserving product integrity across various applications, its safety and environmental implications cannot be ignored. The ongoing dialogue between manufacturers, regulatory bodies, and consumers will shape the future of preservatives in our everyday products. As we move towards a more health-conscious and environmentally friendly paradigm, the industry’s ability to adapt and innovate will be essential in ensuring the safety and efficacy of personal care and household products.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





