Jul . 02, 2024 03:30
Back to list
Exploring the Structure of Polyaspartic Acid A Comprehensive Analysis
Polyaspartic acid, also known as polyaspartate or PASP, is a biodegradable polymer that has attracted significant attention in recent years due to its unique structure and properties. This article will explore the structure of polyaspartic acid and its potential applications in various fields.
The structure of polyaspartic acid is characterized by its repeating units of aspartic acid, which are linked together through amide bonds. The presence of two carboxyl groups in each monomer unit makes it an excellent candidate for forming strong hydrogen bonds with other molecules. This property allows polyaspartic acid to form stable complexes with metal ions, such as calcium and magnesium, making it useful in water treatment and mineral processing industries.
Moreover, the high density of carboxyl groups in polyaspartic acid makes it highly hydrophilic, allowing it to interact effectively with water molecules. This characteristic enables polyaspartic acid to be used as a thickener, stabilizer, or emulsifier in food products, cosmetics, and pharmaceuticals. Additionally, the biodegradability of polyaspartic acid makes it an attractive alternative to traditional petroleum-based polymers in these applications.
In the field of medicine, polyaspartic acid has shown promise as a drug delivery system due to its ability to form nanoparticles that can encapsulate and release drugs in a controlled manner. The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials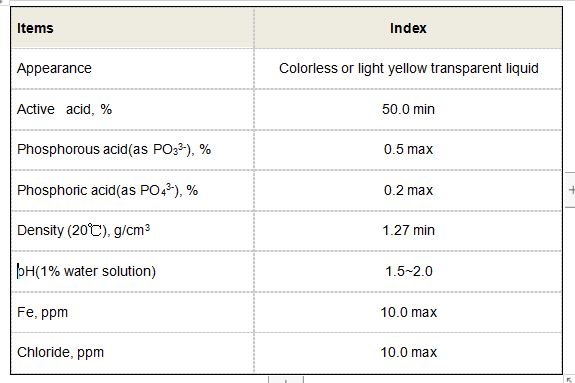 The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials
The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials polyaspartic acid structure. Furthermore, the modular nature of polyaspartic acid allows for easy modification of its structure to tailor its properties for specific applications.
Another potential application of polyaspartic acid is in environmental remediation. Its ability to form stable complexes with heavy metals makes it an ideal candidate for removing these pollutants from wastewater and soil. Additionally, the biodegradability of polyaspartic acid ensures that it does not contribute to further pollution once it has served its purpose.
In conclusion, polyaspartic acid is a versatile polymer with a unique structure that offers numerous potential applications in various fields. Its ability to form strong hydrogen bonds, interact with water molecules, and complex with metal ions makes it a valuable material for use in industries ranging from food and cosmetics to medicine and environmental remediation. As research continues on this fascinating polymer, we can expect to see even more innovative uses for polyaspartic acid in the future.
polyaspartic acid structure. Furthermore, the modular nature of polyaspartic acid allows for easy modification of its structure to tailor its properties for specific applications.
Another potential application of polyaspartic acid is in environmental remediation. Its ability to form stable complexes with heavy metals makes it an ideal candidate for removing these pollutants from wastewater and soil. Additionally, the biodegradability of polyaspartic acid ensures that it does not contribute to further pollution once it has served its purpose.
In conclusion, polyaspartic acid is a versatile polymer with a unique structure that offers numerous potential applications in various fields. Its ability to form strong hydrogen bonds, interact with water molecules, and complex with metal ions makes it a valuable material for use in industries ranging from food and cosmetics to medicine and environmental remediation. As research continues on this fascinating polymer, we can expect to see even more innovative uses for polyaspartic acid in the future.
 The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials
The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials The biocompatibility and biodegradability of polyaspartic acid make it suitable for use in vivo, reducing the risk of adverse reactions or toxicity associated with synthetic materials polyaspartic acid structure. Furthermore, the modular nature of polyaspartic acid allows for easy modification of its structure to tailor its properties for specific applications.
Another potential application of polyaspartic acid is in environmental remediation. Its ability to form stable complexes with heavy metals makes it an ideal candidate for removing these pollutants from wastewater and soil. Additionally, the biodegradability of polyaspartic acid ensures that it does not contribute to further pollution once it has served its purpose.
In conclusion, polyaspartic acid is a versatile polymer with a unique structure that offers numerous potential applications in various fields. Its ability to form strong hydrogen bonds, interact with water molecules, and complex with metal ions makes it a valuable material for use in industries ranging from food and cosmetics to medicine and environmental remediation. As research continues on this fascinating polymer, we can expect to see even more innovative uses for polyaspartic acid in the future.
polyaspartic acid structure. Furthermore, the modular nature of polyaspartic acid allows for easy modification of its structure to tailor its properties for specific applications.
Another potential application of polyaspartic acid is in environmental remediation. Its ability to form stable complexes with heavy metals makes it an ideal candidate for removing these pollutants from wastewater and soil. Additionally, the biodegradability of polyaspartic acid ensures that it does not contribute to further pollution once it has served its purpose.
In conclusion, polyaspartic acid is a versatile polymer with a unique structure that offers numerous potential applications in various fields. Its ability to form strong hydrogen bonds, interact with water molecules, and complex with metal ions makes it a valuable material for use in industries ranging from food and cosmetics to medicine and environmental remediation. As research continues on this fascinating polymer, we can expect to see even more innovative uses for polyaspartic acid in the future. Share
Latest news
-
Scale and Corrosion Inhibitors: Key to Industrial Water TreatmentNewsMay.22,2025
-
Organic Phosphate: Structure, Properties, and ApplicationsNewsMay.22,2025
-
Isothiazolinones: a versatile and versatile biocide with a wide range of applicationsNewsMay.22,2025
-
Industrial Flocculant: The Key to Optimizing Industrial ProcessesNewsMay.22,2025
-
Hydrolyzed Polymaleic Anhydride: Structure, Properties, and ApplicationsNewsMay.22,2025
-
Application of Flocculant in Water TreatmentNewsMay.22,2025





