Effective Strategies for Corrosion Scale Control and Inhibition in Various Industries
Understanding Corrosion Scale Inhibitors A Key to Enhanced Asset Longevity
Corrosion is a natural process that gradually deteriorates materials, particularly metals, through their reaction with environmental elements such as moisture, oxygen, and salts. This deterioration can lead to significant economic losses, operational disruptions, and safety hazards, especially in industrial settings where metal components are pivotal. To combat this pervasive issue, corrosion scale inhibitors have emerged as a crucial line of defense.
What are Corrosion Scale Inhibitors?
Corrosion scale inhibitors are chemical substances designed to prevent or slow down the corrosion process by forming a protective layer on metal surfaces or by modifying the environment around them. They are widely used in various industries, including oil and gas, water treatment, power generation, and manufacturing. By incorporating these inhibitors, companies can extend the operational life of critical equipment and reduce maintenance costs significantly.
Mechanisms of Action
Corrosion scale inhibitors operate through various mechanisms, which can be broadly categorized into two main types anodic inhibitors and cathodic inhibitors.
1. Anodic Inhibitors These inhibitors work by decreasing the rate of oxidation at the anode of the corrosion cell. They promote the formation of a passivation layer, also known as a protective oxide layer, which deters further corrosion. Common anodic inhibitors include chromates, phosphates, and molybdates.
2. Cathodic Inhibitors These inhibitors impede the reduction reactions that occur at the cathode. By suppressing these reactions, they effectively slow down the overall corrosion process. Examples of cathodic inhibitors include zinc and barium compounds, which can help mitigate the effects of localized corrosion.
Types of Corrosion Scale Inhibitors
Corrosion scale inhibitors can also be classified based on their chemical composition
corrosion scale inhibitor
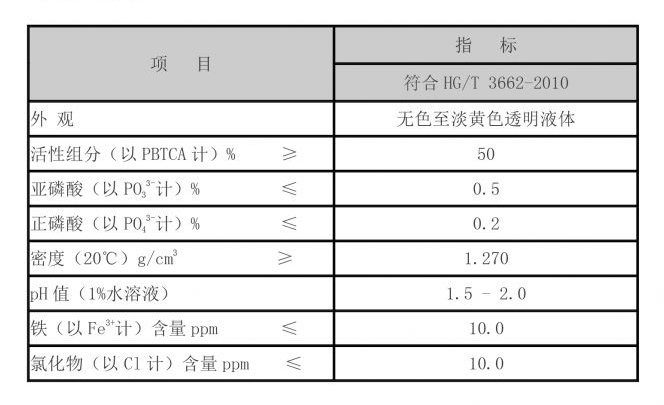
- Organic Inhibitors These are often derived from natural compounds (such as plant extracts) or synthetic chemicals (like amines and surfactants). They tend to form protective films on metal surfaces and are favored in many applications due to their effectiveness and lower toxicity.
- Inorganic Inhibitors Commonly used inorganic inhibitors, such as phosphonates and silicates, can effectively reduce corrosion in various environments. Their ability to bond with metal surfaces makes them ideal for many industrial applications.
Benefits of Using Corrosion Scale Inhibitors
1. Cost-Efficiency By preserving the integrity of equipment and infrastructure, corrosion scale inhibitors can significantly lower maintenance and replacement costs.
2. Safety Reducing corrosion risk ensures safer operational conditions, minimizing the likelihood of leaks and catastrophic failures within industrial systems.
3. Environmental Protection Many corrosion inhibitors have been developed with eco-friendly formulations that reduce environmental toxicity and promote sustainability.
4. Enhanced Performance The use of corrosion scale inhibitors can lead to improved efficiency in operational processes by preventing unplanned downtime due to equipment failures.
Conclusion
Corrosion is an ever-present threat in many industries, but the application of corrosion scale inhibitors presents a viable solution for mitigating its impact. By understanding the types of inhibitors available and their mechanisms of action, industries can better protect their assets, enhance operational longevity, and promote safety. The ongoing research and development in this field continue to introduce innovative inhibitors, adapting to emerging challenges and environmental regulations. As we progress into a more sustainability-focused future, the role of corrosion scale inhibitors will undoubtedly become even more critical in the fight against material degradation, securing both economic and environmental benefits for industries worldwide.
-
Scale and Corrosion Inhibitors: Key to Industrial Water TreatmentNewsMay.22,2025
-
Organic Phosphate: Structure, Properties, and ApplicationsNewsMay.22,2025
-
Isothiazolinones: a versatile and versatile biocide with a wide range of applicationsNewsMay.22,2025
-
Industrial Flocculant: The Key to Optimizing Industrial ProcessesNewsMay.22,2025
-
Hydrolyzed Polymaleic Anhydride: Structure, Properties, and ApplicationsNewsMay.22,2025
-
Application of Flocculant in Water TreatmentNewsMay.22,2025





