Exploring the Impacts of HEDP in Various Industrial Applications and Processes
Exploring the Implications of HEDP and pH An Integral Relationship in Water Chemistry
In the realm of water treatment and chemical analysis, the interaction between chemical agents and environmental conditions plays a critical role in effective management. One such agent gaining attention is Hydroxyethylidene Diphosphonic Acid (HEDP), a phosphonate compound widely used for its chelating properties. The pH level of a solution significantly influences the effectiveness of HEDP, as well as its environmental behavior and applications in various industries.
Understanding HEDP
HEDP is a versatile chemical known for its ability to inhibit scale formation and corrosion in water systems. It is particularly valuable in industrial applications such as cooling towers, oil drilling, and textile processing. By binding to metal ions, HEDP prevents them from forming insoluble scale deposits, which can impair system efficiency and lead to increased operational costs. The strength of bond formation between HEDP and metal ions, however, is highly dependent on the pH of the solution.
The Role of pH in HEDP Functionality
pH, a measure of the hydrogen ion concentration in a solution, can significantly alter the behavior and efficacy of HEDP. At lower pH levels, HEDP exists in a more protonated form, which can reduce its ability to chelate metal ions effectively. This is due to the competing hydrogen ions that occupy binding sites on the HEDP molecule, thereby decreasing its reactivity. Conversely, at higher pH levels, HEDP becomes deprotonated, enhancing its chelating ability and allowing for stronger interactions with various metal cations such as calcium, magnesium, and iron.
hedp ph
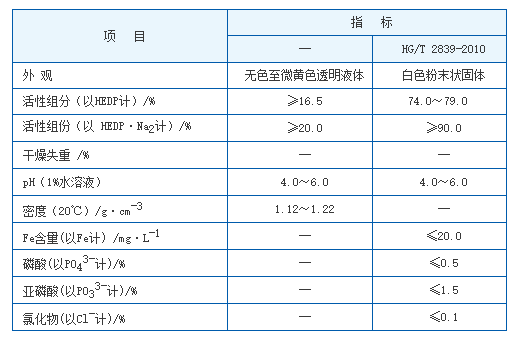
Optimal pH Levels for HEDP Use
The optimal pH level for utilizing HEDP generally falls within a range of 6 to 10, where it exhibits strong chelation capabilities. In cooling water systems, maintaining a balanced pH is crucial for maximizing HEDP's performance. When the pH deviates from this optimal range, the efficacy of HEDP diminishes, leading to potential scale formation and reduced corrosion protection. Therefore, regular monitoring and adjustment of pH levels in water treatment processes are necessary to ensure the continued effectiveness of HEDP as a scale and corrosion inhibitor.
Implications for Use in Industry
Industries that rely on HEDP must consider pH not only for its chemical efficacy but also from an environmental standpoint. The release of HEDP into the environment can have implications for aquatic ecosystems, especially in cases where pH levels are altered by industrial discharge. Studies have shown that the breakdown products of HEDP in various pH conditions can exhibit different degrees of biodegradability and toxicity. Hence, understanding the interaction between HEDP and pH is vital for developing strategies to minimize environmental impact while ensuring industrial efficiency.
Conclusion
The relationship between HEDP and pH is a fundamental aspect of water chemistry that influences the performance of treatment processes across various industries. To harness the full potential of HEDP, it is essential to maintain optimal pH levels, thereby enhancing chelation and corrosion inhibition. Furthermore, recognizing the environmental implications of HEDP usage highlights the need for responsible management and sustainable practices in industrial applications. As the demand for efficient water treatment solutions continues to grow, the importance of understanding the interplay between HEDP and pH will only increase, driving innovation and improvements in water chemistry.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





