polycarboxylic
Understanding Polycarboxylic Compounds Structures, Properties, and Applications
Polycarboxylic compounds are a fascinating class of organic molecules characterized by the presence of multiple carboxyl (-COOH) functional groups in their structure. These compounds have garnered significant interest in various fields, including chemistry, materials science, and biology, due to their unique properties and versatile applications.
Structure and Classification
Polycarboxylic acids can be classified based on the number of carboxyl groups they possess. For instance, dicarboxylic acids contain two carboxyl groups, while tricarboxylic acids have three, and so forth. Common examples include oxalic acid (H2C2O4), which is a dicarboxylic acid, and citric acid (C6H8O7), a tricarboxylic acid widely found in citrus fruits.
The presence of multiple carboxyl groups distinguishes polycarboxylic acids from their monocarboxylic counterparts and plays a crucial role in their chemical behavior. The carboxyl groups can participate in various chemical reactions, making these compounds vital intermediates in organic synthesis.
Properties of Polycarboxylic Compounds
One of the most notable properties of polycarboxylic compounds is their acidity. The presence of multiple carboxyl groups leads to an increased capacity to donate protons (H+), which makes these acids significantly stronger than their monocarboxylic analogs. For example, citric acid, with three carboxyl groups, is a weak acid that can dissociate in solutions, contributing to its sour taste in foods and beverages.
polycarboxylic
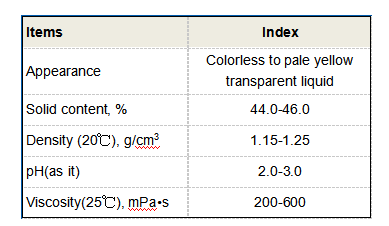
Moreover, polycarboxylic acids possess a high degree of polarity due to the electronegative oxygen atoms present in the carboxyl groups
. This polarity renders them highly soluble in water, which enhances their reactivity and ability to form complexes with metal ions. The solubility and reactivity of these compounds make them suitable candidates as chelating agents, stabilizing metal ions in solution.Applications in Various Fields
Polycarboxylic compounds have a wide range of applications across different sectors. In the field of materials science, they are often used as building blocks for polymers. Polyacrylic acid, for instance, is a polycarboxylic polymer that exhibits excellent adhesive properties and is used in the formulation of superabsorbent materials such as diapers.
In the realm of agriculture, polycarboxylic acids can function as soil conditioners and chelating agents that enhance the availability of essential nutrients to plants. For example, humic and fulvic acids, which are derived from decomposed organic matter, are complex mixtures of polycarboxylic acids that improve soil structure and fertility.
Furthermore, polycarboxylic acids have shown promise in medical applications. Some polycarboxylic derivatives are exploited as drug delivery systems due to their ability to form nanoparticles, encapsulating therapeutic agents and facilitating their release in targeted areas of the body. Additionally, their biocompatibility and biodegradability make them suitable candidates for developing new biomaterials for tissue engineering and regenerative medicine.
Conclusion
Polycarboxylic compounds are essential entities in both nature and synthetic chemistry. Their unique structural features give rise to diverse physical and chemical properties that make them invaluable in various industrial applications. From enhancing agricultural productivity to driving innovations in drug delivery systems, the significance of polycarboxylic compounds continues to expand. As research progresses, the potential for new applications of these versatile molecules is bound to grow, unlocking further possibilities for their use in everyday life and technology. Understanding and harnessing the properties of polycarboxylic acids will undoubtedly play a pivotal role in the advancements of future scientific endeavors.
-
Understanding Polycarboxylic Acids: Properties, Applications, and Future PotentialNewsJul.28,2025
-
Scale Inhibitor Explained: How to Protect Your System from Limescale and Hard Water DamageNewsJul.28,2025
-
Scale and Corrosion Inhibitors: Essential Chemicals for Industrial Water System ProtectionNewsJul.28,2025
-
Polyaspartic Acid: A Biodegradable Polymer for Sustainable ChemistryNewsJul.28,2025
-
Isothiazolinones: A Versatile Antimicrobial Class with Industrial Power and Regulatory ChallengesNewsJul.28,2025
-
A Deep Dive into 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTC)NewsJul.28,2025





