Butane-1,2,4-Tricarboxylic Acid and Its Potential Applications in Organic Chemistry
The Importance of Butane-1,2,4-Tricarboxylic Acid A Comprehensive Overview
Butane-1,2,4-tricarboxylic acid (BTA) is a triprotic carboxylic acid that plays a significant role in various chemical and biological processes. Its unique structure and functional groups make it an important compound in organic chemistry, with numerous applications ranging from pharmaceuticals to agriculture. This article delves into the properties, synthesis, and applications of butane-1,2,4-tricarboxylic acid, highlighting its relevance in contemporary science and industry.
Chemical Structure and Properties
Butane-1,2,4-tricarboxylic acid is characterized by three carboxylic acid groups (-COOH) attached to a butane backbone. This unique arrangement of carboxyl groups allows BTA to engage in multiple protonation states, making it versatile in various chemical reactions. The presence of three carboxylic acid groups enhances its acidity and reactivity, which can be exploited in synthesis and catalysis.
BTA appears as a crystalline solid at room temperature and is soluble in water and organic solvents. Its melting point and other physical properties are indicative of its hydrogen bonding capabilities and the strength of intermolecular forces. These properties make butane-1,2,4-tricarboxylic acid a significant compound in a range of chemical contexts.
Synthesis of Butane-1,2,4-Tricarboxylic Acid
The synthesis of butane-1,2,4-tricarboxylic acid can be achieved through several pathways, with the most common method involving the oxidation of butanes or other readily available hydrocarbon precursors. One approach involves the oxidative cleavage of a polyunsaturated compound like fumaric acid or maleic acid, which subsequently leads to the formation of BTA through a series of chemical transformations.
Another interesting method is the use of microbial fermentation processes that leverage specific bacteria capable of converting simple carbohydrates or butanes into BTA. This biotechnological approach is gaining traction, making the synthesis of BTA more sustainable and environmentally friendly compared to traditional chemical methods.
butane 1 2 4 tricarboxylic acid
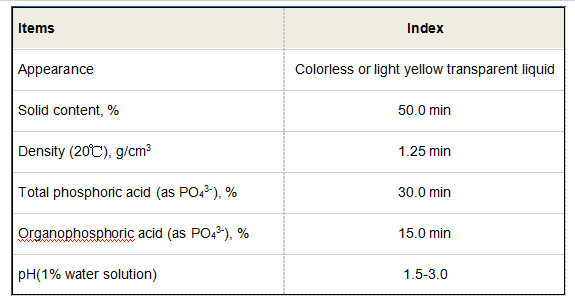
Applications of Butane-1,2,4-Tricarboxylic Acid
1. Pharmaceutical Industry BTA is involved in the synthesis of various pharmaceutical agents. Its ability to form chelates with metal ions is particularly useful in drug formulation, enhancing the bioavailability of certain compounds. Additionally, the acid acts as an intermediate in the production of more complex molecules, such as anti-inflammatory drugs and other therapeutic agents.
2. Agricultural Uses Butane-1,2,4-tricarboxylic acid is also finding applications in agriculture. As a natural chelating agent, it helps in improving nutrient absorption in plants by binding essential minerals. This property is particularly beneficial in enhancing soil quality and promoting crop yields, making it a valuable tool for sustainable agriculture.
3. Food Industry In the food sector, BTA serves as a food additive and preservative. Its ability to inhibit microbial growth helps in prolonging the shelf life of food products. Additionally, it can assist in pH regulation, providing stability in various food formulations.
4. Chemical Research Researchers are continuously exploring new applications of BTA in catalysts and as a precursor for advanced materials. Its multifunctionality and ability to act as a linker in polymer chemistry are invaluable for developing novel materials with tailored properties.
Conclusion
Butane-1,2,4-tricarboxylic acid is an essential compound in various fields due to its unique chemical properties and versatile applications. As research continues to uncover new uses and synthesis methods, BTA stands as an exemplary molecule that bridges multiple disciplines, including chemistry, agriculture, and pharmaceuticals. The ongoing exploration of this compound underscores the importance of understanding and innovating with such critical organic acids in both scientific and industrial contexts. As we advance into an era of heightened focus on sustainability and efficiency, butane-1,2,4-tricarboxylic acid is poised to play an even more integral role in addressing global challenges.
-
Understanding Polycarboxylic Acids: Properties, Applications, and Future PotentialNewsJul.28,2025
-
Scale Inhibitor Explained: How to Protect Your System from Limescale and Hard Water DamageNewsJul.28,2025
-
Scale and Corrosion Inhibitors: Essential Chemicals for Industrial Water System ProtectionNewsJul.28,2025
-
Polyaspartic Acid: A Biodegradable Polymer for Sustainable ChemistryNewsJul.28,2025
-
Isothiazolinones: A Versatile Antimicrobial Class with Industrial Power and Regulatory ChallengesNewsJul.28,2025
-
A Deep Dive into 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTC)NewsJul.28,2025





