Exploring the Characteristics and Applications of CAS 207414 83 7 in Chemical Research
Understanding CAS Number 7414-83-7 A Comprehensive Overview
The world of chemistry is often filled with complexities, particularly when it comes to the identification and classification of chemical substances. One of the key tools in this realm is the Chemical Abstracts Service (CAS) Registry Number, a unique numerical identifier that provides a means for chemists and researchers to communicate clearly about specific chemicals without confusion. This article will delve into the significance of the CAS number 7414-83-7, its chemical composition, uses, safety protocols, and its role in various industries.
CAS number 7414-83-7 corresponds to a compound known as Sodium Sulfide, specifically its anhydrous form. Sodium sulfide is an inorganic compound with the formula Na2S. It appears as a white to yellowish solid and is highly soluble in water. Sodium sulfide is classified as an alkaline sulfide due to the presence of sulfide ions (S^2−). The anhydrous nature of this compound refers to its lack of water molecules, which can influence its properties and uses.
Understanding CAS Number 7414-83-7 A Comprehensive Overview
Sodium sulfide has several applications that range across various fields, including textiles, paper, leather, and mining industries. In the textile industry, it is primarily used in the dyeing process, especially for the production of vat dyes, which are insoluble in water and require reduction to a soluble form for application. This chemical acts as a reducing agent which helps in converting the insoluble dye into a soluble form, allowing it to be absorbed by fabric.
cas 7414 83 7
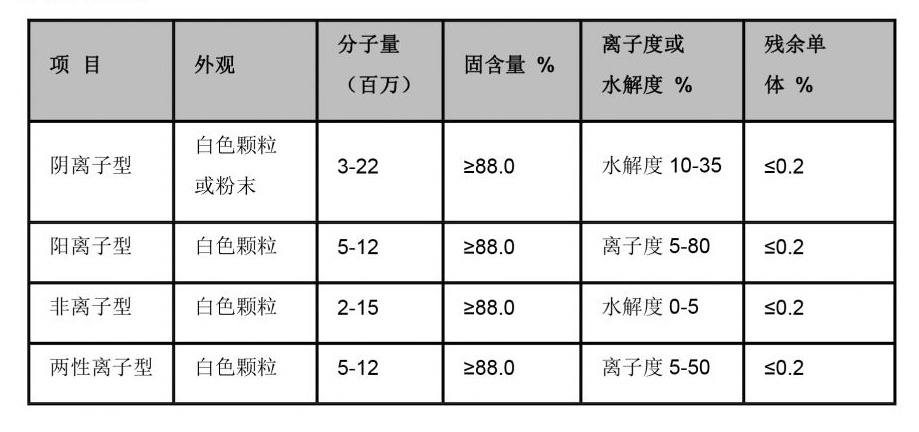
In the paper and pulp industry, sodium sulfide is utilized during the kraft pulping process. It plays an essential role in breaking down lignin, which binds cellulose fibers together. The removal of lignin improves the yield and quality of cellulose, leading to higher quality paper products. Furthermore, the leather industry utilizes sodium sulfide for the depilation of hides, removing hair and other unwanted materials before tanning.
While sodium sulfide has many beneficial applications, it is essential to acknowledge its hazardous nature. It is classified as a toxic and corrosive substance, posing risks to human health and the environment. Exposure to sodium sulfide can lead to irritation of the skin, eyes, and respiratory tract. Moreover, it can release harmful hydrogen sulfide gas (H2S) when in contact with acids, a compound that is well-known for its noxious odor and toxic properties. Thus, proper safety protocols must be in place when handling this chemical. Protective equipment such as gloves, goggles, and respiratory protection should be worn to prevent exposure. Additionally, adequate ventilation and awareness of emergency procedures are crucial to mitigate risks associated with accidental spills or exposure.
From a regulatory standpoint, the use of sodium sulfide is governed by various environmental and safety agencies, which emphasize the importance of conducting risk assessments and adhering to guidelines for safe handling and disposal. The challenges posed by its toxicity necessitate ongoing research into safer alternatives, as well as improved methods for the management of waste containing sodium sulfide.
In conclusion, CAS number 7414-83-7 represents sodium sulfide, an important inorganic compound with wide-ranging applications. While it serves crucial functions in various industries, it is imperative to recognize the associated health and environmental risks. Through careful handling, adherence to safety regulations, and continued research, the benefits of sodium sulfide can be harnessed while minimizing its potential hazards. Understanding the chemical nature and applications of such compounds ensures that advancements in technology and industry are pursued safely and responsibly.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





