Exploring the Role of PBTC in Enhancing Tricarboxylic Acid Cycle Efficiency and Metabolic Pathways
Understanding PBTC and Its Role in the Tricarboxylic Acid Cycle
The tricarboxylic acid (TCA) cycle, also known as the citric acid cycle or Krebs cycle, is a crucial metabolic pathway that plays a central role in cellular respiration. It facilitates the conversion of carbohydrates, fats, and proteins into carbon dioxide and water, releasing energy stored in chemical bonds. Among various compounds involved in this intricate process, PBTC (phosphonobutane tricarboxylic acid) has garnered attention for its unique properties and potential applications.
The Basics of the TCA Cycle
Before delving into PBTC, it’s essential to understand the TCA cycle’s significance. Located in the mitochondria of eukaryotic cells, the TCA cycle is vital for producing ATP (adenosine triphosphate), the energy currency of the cell. The cycle begins with the condensation of acetyl-CoA and oxaloacetate, resulting in citric acid. As the cycle progresses, citric acid undergoes a series of transformations, eventually regenerating oxaloacetate. During these transformations, carbon dioxide is released, and high-energy carriers such as NADH and FADH2 are generated.
Introduction to PBTC
PBTC is an organic compound that could influence various biochemical processes. It is a phosphonate compound that can act as a ligating agent, similar to citric acid, which is naturally occurring in the TCA cycle. PBTC’s structure, featuring three carboxylic acid groups attached to a butane backbone, makes it a unique player in biochemical interactions.
One of the intriguing aspects of PBTC is its potential to enhance the efficiency of enzyme reactions associated with the TCA cycle. By modulating the availability of metal ions that serve as cofactors for dehydrogenase enzymes, PBTC can affect the overall energy production within cells. The presence of tricarboxylic acid groups allows PBTC to chelate metal ions, thereby influencing metabolic pathways.
pbtc tricarboxylic acid
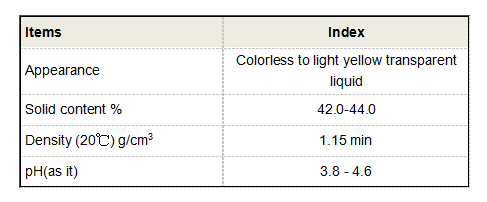
Potential Applications of PBTC
The role of PBTC is not restricted solely to the cell's metabolism. Researchers are also exploring its applications in agriculture, pharmaceuticals, and environmental science. In agriculture, for instance, PBTC serves as a biodegradable chelating agent, aiding in the delivery of essential micronutrients to plants. By improving nutrient availability, PBTC can enhance plant growth and yield.
In the pharmaceutical industry, PBTC's chelating properties may lead to novel drug formulations that target specific diseases related to metal ion imbalances. By facilitating the transport and stability of certain drugs, PBTC could help improve treatment outcomes.
Furthermore, its potential in environmental science is promising. PBTC can be used in water treatment processes to bind heavy metals, thus reducing their bioavailability and toxicity in ecosystems.
Conclusion
In conclusion, PBTC represents a multifaceted compound with significant implications for both biological processes and practical applications. Its relationship with the TCA cycle underscores the intricate connections among metabolic pathways and the various compounds that influence them. As research continues, the potential for PBTC to impact agriculture, pharmaceuticals, and environmental protection illustrates the relevance and importance of understanding such compounds in a comprehensive manner. Overall, the study of PBTC and its interactions within metabolic processes highlights the ongoing exploration of biochemical compounds as vital tools for innovation and sustainability.
-
Water Treatment with Flocculant Water TreatmentNewsJun.12,2025
-
Polymaleic AnhydrideNewsJun.12,2025
-
Polyaspartic AcidNewsJun.12,2025
-
Enhance Industrial Processes with IsothiazolinonesNewsJun.12,2025
-
Enhance Industrial Processes with PBTCA SolutionsNewsJun.12,2025
-
Dodecyldimethylbenzylammonium Chloride SolutionsNewsJun.12,2025





