Synthesis and Characterization of Butane-1,2,4-Tricarboxylic Acid Derivatives for Potential Applications
The Role of Butane-1,2,4-Tricarboxylic Acid in Organic Chemistry
Butane-1,2,4-tricarboxylic acid, often referred to as citraconic acid due to its structural affinity with citric acid albeit lacking one carboxyl group, is a notable compound in organic chemistry. Its significance arises from its versatile applications in chemical synthesis, industrial processes, and its relevance in biological systems. This article aims to explore the properties, synthesis, and applications of butane-1,2,4-tricarboxylic acid.
Properties
Butane-1,2,4-tricarboxylic acid is a tricarboxylic acid characterized by three carboxyl functional groups (-COOH) attached to a four-carbon alkane chain. The molecular formula for this compound is C7H10O6, and it typically appears as a white crystalline solid at room temperature. Its structural formula allows it to exhibit an interesting acidity profile, making it a suitable candidate for various chemical reactions. The compound is soluble in water and has a relatively low melting point, which contributes to its ease of handling in laboratory and industrial environments.
The Role of Butane-1,2,4-Tricarboxylic Acid in Organic Chemistry
The synthesis of butane-1,2,4-tricarboxylic acid can be approached through different routes, with one of the most prominent being the malonic acid condensation reaction. Starting with malonic acid, a well-known dicarboxylic acid, and utilizing a series of controlled reactions involving acetic anhydride or other suitable reagents, chemists can effectively construct the tricarboxylic acid framework. The end product can be purified through crystallization techniques to ensure the removal of any residual reagents or by-products, resulting in a high-purity compound ready for research or industrial use.
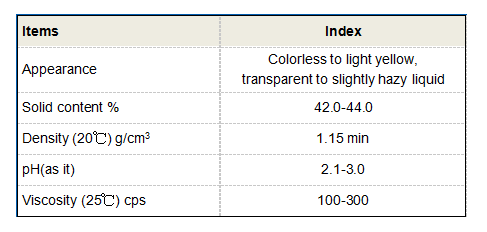
butane 1 2 4 tricarboxylic acid
Applications
Butane-1,2,4-tricarboxylic acid finds its utility in several domains. In organic synthesis, it serves as a building block for more complex molecules. For instance, its multiple carboxyl groups allow it to participate in esterification reactions, leading to the production of esters that can be valuable in various applications such as in the manufacturing of plasticizers, solvents, and fragrances. The carboxylic acid functionality also makes it a reactive species in polymer chemistry, where it can be used to modify the properties of polymers or as a crosslinking agent.
Moreover, the biological implications of butane-1,2,4-tricarboxylic acid cannot be overlooked. Compounds structurally related to it are often encountered in metabolic pathways, particularly in the context of the citric acid cycle, a fundamental biochemical pathway that generates energy in aerobic organisms. While butane-1,2,4-tricarboxylic acid may not directly participate in these pathways, its derivatives and related compounds are crucial for maintaining metabolic balance and energy production within cells.
Conclusion
In summary, butane-1,2,4-tricarboxylic acid serves as an important compound in both organic chemistry and biological contexts. Its unique structure, coupled with its reactive nature, allows it to play a significant role in various chemical synthesis processes and applications. As research continues to evolve, the understanding of its properties and potential applications may lead to the development of new materials and methods that enhance our capabilities in both industrial and scientific fields. The continued exploration of such compounds underscores the importance of organic chemistry in addressing modern challenges, from energy production to the development of new materials.
-
Pbtc Scale InhibitorPBTC: A Scale Protector for Industrial Water TreatmentNewsAug.05,2025
-
Organic Phosphonate: An Efficient Defender in the Field of Scale InhibitionNewsAug.05,2025
-
Hydrolyzed Polymaleic Anhydride: Green Pioneer in Scale Inhibition FieldNewsAug.05,2025
-
PAPEMP Polyamino Polyether Methylene Phosphonic Acid For SaleNewsAug.05,2025
-
Flocculant Water Treatment: A Pioneer in Purification in the Field of Water TreatmentNewsAug.05,2025
-
Benzyl Isothiazolinone: An Efficient and Broad-Spectrum Antibacterial Protective GuardNewsAug.05,2025





